Question: The irreversible zero-order reaction A B is taking place inside the porous catalyst slab shown in Fig 1. [A]o [A]0 [A] = f(x) 2L
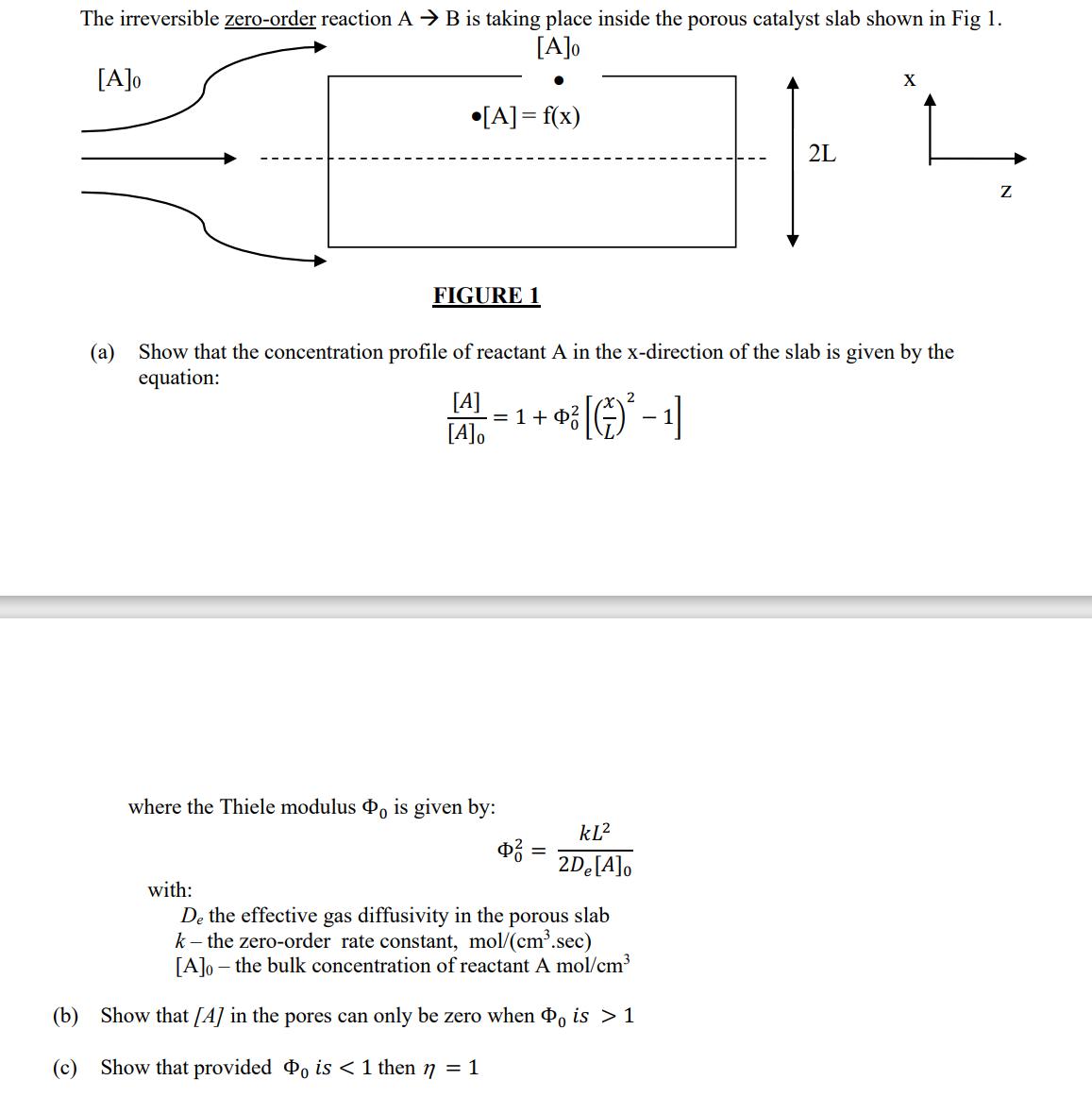
The irreversible zero-order reaction A B is taking place inside the porous catalyst slab shown in Fig 1. [A]o [A]0 [A] = f(x) 2L X FIGURE 1 (a) Show that the concentration profile of reactant A in the x-direction of the slab is given by the equation: [A] -= 1 + [A]0 2 + } [() - 1] where the Thiele modulus Oo is given by: 0 with: KL ? - 2De[A]o De the effective gas diffusivity in the porous slab k-the zero-order rate constant, mol/(cm.sec) [A]o the bulk concentration of reactant A mol/cm (b) Show that [A] in the pores can only be zero when o is > 1 (c) Show that provided Po is < 1 then 17 = 1 Z
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


