Question: 5. Data below (also in Brightspace) indicate freezing point depression (compared to pure water) for dilute aque- ous solutions of either NaCl or MgSO4.
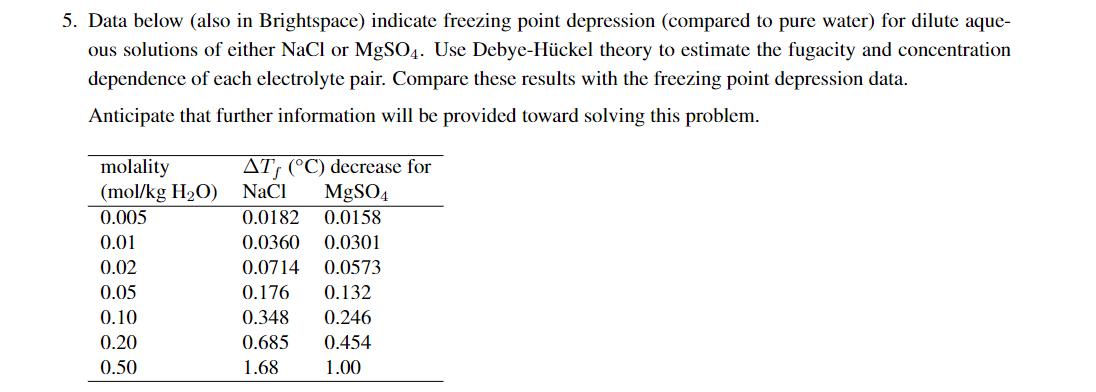
5. Data below (also in Brightspace) indicate freezing point depression (compared to pure water) for dilute aque- ous solutions of either NaCl or MgSO4. Use Debye-Hckel theory to estimate the fugacity and concentration dependence of each electrolyte pair. Compare these results with the freezing point depression data. Anticipate that further information will be provided toward solving this problem. molality (mol/kg H2O) AT (C) decrease for NaCl MgSO4 0.005 0.0182 0.0158 0.01 0.0360 0.0301 0.02 0.0714 0.0573 0.05 0.176 0.132 0.10 0.348 0.246 0.20 0.685 0.454 0.50 1.68 1.00
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


