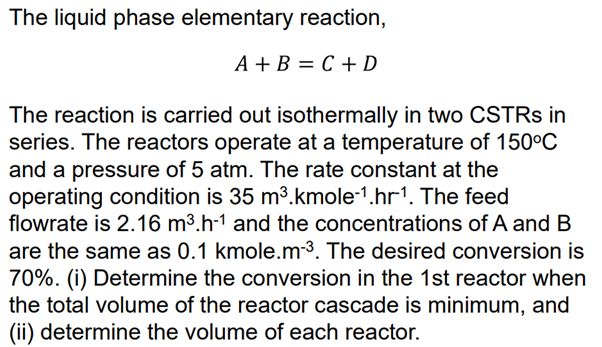
Question: The liquid phase elementary reaction, A + B = C + D The reaction is carried out isothermally in two CSTRs in series. The reactors
The liquid phase elementary reaction,
The reaction is carried out isothermally in two CSTRs in
series. The reactors operate at a temperature of
and a pressure of atm. The rate constant at the
operating condition is The feed
flowrate is and the concentrations of A and
are the same as kmole. The desired conversion is
i Determine the conversion in the st reactor when
the total volume of the reactor cascade is minimum, and
ii determine the volume of each reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


