Question: (30 points) For a reaction, A B + C, is to be carried out in a continuous-flow reactor. Assume the reaction rate - 1
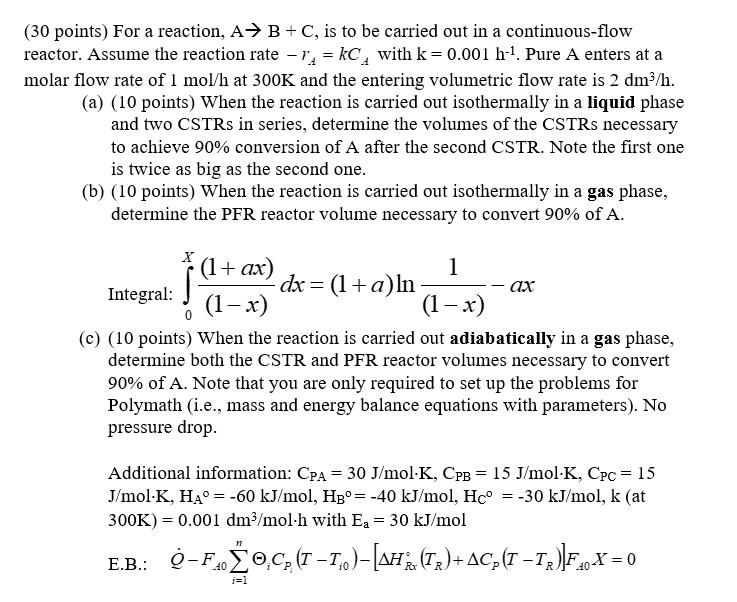
(30 points) For a reaction, A B + C, is to be carried out in a continuous-flow reactor. Assume the reaction rate - 1 = kC with k = 0.001 h-. Pure A enters at a molar flow rate of 1 mol/h at 300K and the entering volumetric flow rate is 2 dm/h. (a) (10 points) When the reaction is carried out isothermally in a liquid phase and two CSTRS in series, determine the volumes of the CSTRS necessary to achieve 90% conversion of A after the second CSTR. Note the first one is twice as big as the second one. (b) (10 points) When the reaction is carried out isothermally in a gas phase, determine the PFR reactor volume necessary to convert 90% of A. X (1+ax) 1 Integral: dx = (1+a)ln ax (1-x) (1-x) (c) (10 points) When the reaction is carried out adiabatically in a gas phase, determine both the CSTR and PFR reactor volumes necessary to convert 90% of A. Note that you are only required to set up the problems for Polymath (i.e., mass and energy balance equations with parameters). No pressure drop. Additional information: CPA = 30 J/mol-K, CPB = 15 J/mol-K, Cpc = 15 J/mol-K, HA = -60 kJ/mol, HB= -40 kJ/mol, Hc = -30 kJ/mol, k (at 300K) 0.001 dm/mol-h with E = 30 kJ/mol = 12 E.B.: -F,C (T-I)-[AH(Tx)+ AC(T T)]F 4 X = 0 40 R 40
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


