Question: The liquid phase reaction given below A+B2C is carried out adiabatically in a CSTR. A and B are fed to the reactor at 200C together
The liquid phase reaction given below

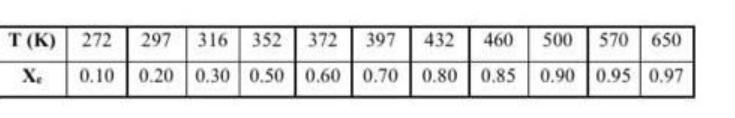
H (273 K) = -33.5 kJ/mol HB (273 K) = -41.5 kJ/mol H(273 K) = -15.6 kJ/mol HD (273 K) = -9.5 kJ/mol Cps = 26.5 J/mol.K CpB = 23.5 J/mol.K Cpc = 25 J/mol.K CpD = 29 J/mol.K k = 1.2 L/mol.min and Kc = 2.1@65C EA = 12.5 kJ/mol Vo = 100 L/min
Step by Step Solution
★★★★★
3.32 Rating (137 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


