Question: The Nernst equation will be applied. E = Eo (0.0592V) logQ Calculate the Eo of the cell. (hint: the oxidation and reduction equations are likely
The Nernst equation will be applied.
E = Eo (0.0592V) logQ
- Calculate the Eo of the cell. (hint: the oxidation and reduction equations are likely to be equal and opposite one another! This is not a trick question.)
- Determine n for this cell. (n = moles of electrons being transferred)
- The concentrations are relative. Use 100, 50, 10. Which is the one in the numerator? The one that produces the sign you saw on your voltmeter.


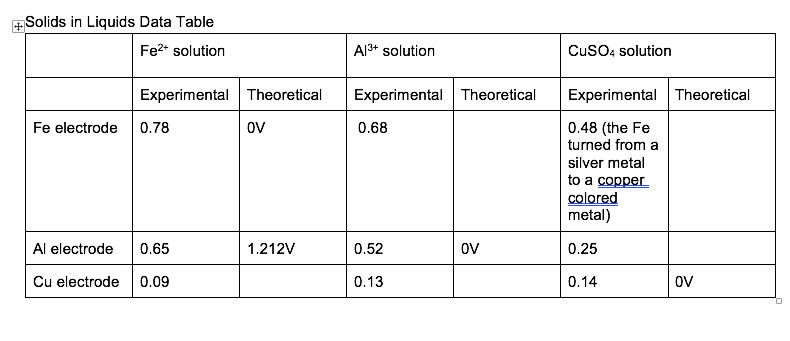
Galvanic cells at 20m 100% - 50% 100% - 10% 50% - 10% 0.00009 0.0003 0.0004 Inert graphite electrodes were used to create (what were the ions) solutions. A voltage of (battery voltage) Volts was applied to (what electrode?) to create this solution in a previously prepared salt water solution. The salt in the water served as (why was there salt?) The voltage was applied for (how long or what criteria was used to stop the reaction?) Galvanic cells of (what?) were tested. Concentration cells were tested and a voltage of was found to be between 100% and 10% solutions. The theoretical value of was found by applying the (what's the name?) Equation. It was determined that the solution was the oxidation and the solution was the reduction. Potential errors in the reporting of the voltages could be (explain potential errors. Potential errors are based on the assumptions made. If you say "human error" or "reading the multimeter" then it is -20 for this lab. Think chemically.) Solids in Liquids Data Table Fe2+ solution A13+ solution CuSO4 solution Experimental Theoretical Experimental Theoretical Fe electrode 0.78 OV 0.68 Experimental Theoretical 0.48 (the Fe turned from a silver metal to a copper colored metal) Al electrode 0.65 1.212V 0.52 OV 0.25 Cu electrode 0.09 0.13 0.14 OV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


