Question: The net ionic equation for the reaction between aqueous nitric acid and aqueous sodium nitrate is A) H+ (aq) + HNO3 (aq) + 2OH- (aq)
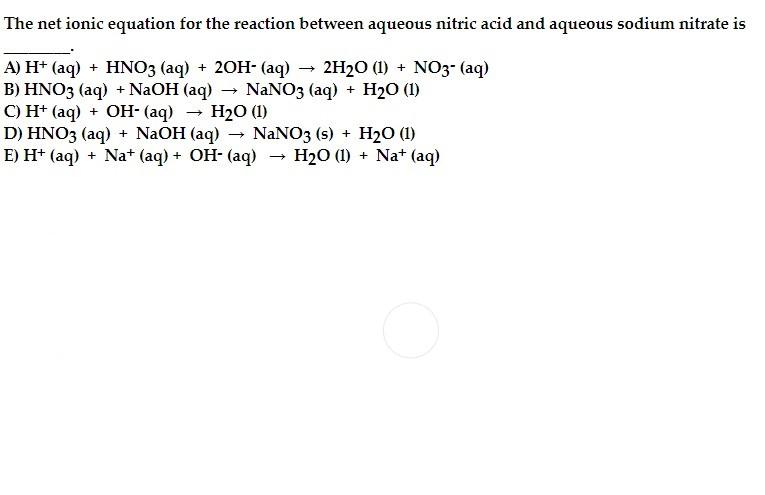
The net ionic equation for the reaction between aqueous nitric acid and aqueous sodium nitrate is A) H+ (aq) + HNO3 (aq) + 2OH- (aq) + 2H20 (1) + NO3- (aq) B) HNO3 (aq) + NaOH(aq) - NaNO3 (aq) + H20 (1) C) H+ (aq) + OH- (aq) H2O (1) D) HNO3 (aq) + NaOH(aq) NaNO3 (s) + H20 (1) E) H+ (aq) + Na+ (aq) + OH- (aq) H20 (1) + Na+ (aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


