Question: The partition coefficient P (or Kow) for a solute between water and n-octanol is defined as the ratio of the solute concentration (moles/volume) in n-octanol
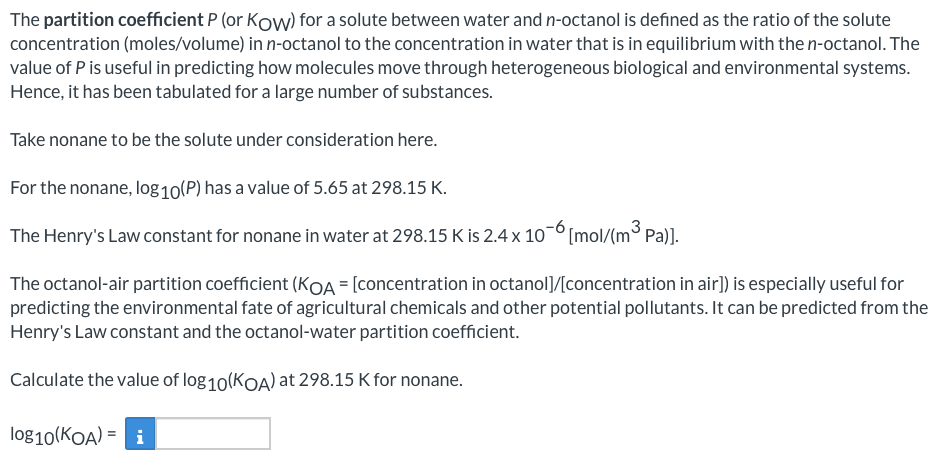
The partition coefficient P (or Kow) for a solute between water and n-octanol is defined as the ratio of the solute concentration (moles/volume) in n-octanol to the concentration in water that is in equilibrium with the n-octanol. The value of P is useful in predicting how molecules move through heterogeneous biological and environmental systems. Hence, it has been tabulated for a large number of substances. Take nonane to be the solute under consideration here. For the nonane, log10(P) has a value of 5.65 at 298.15 K. The Henry's Law constant for nonane in water at 298.15 K is 2.4 x 10-6[mol/(m3 Pa)]. The octanol-air partition coefficient (KOA = [concentration in octanol]/[concentration in air]) is especially useful for predicting the environmental fate of agricultural chemicals and other potential pollutants. It can be predicted from the Henry's Law constant and the octanol-water partition coefficient. Calculate the value of log10(Koa) at 298.15 K for nonane. log10(KOA) =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


