Question: The Quantitative Structure Activity Relationship (QSAR) equation relating the analgesic activity with the Hammet constant (c) and substituent hydrophobicity constant (TT) of the aromatic substituent
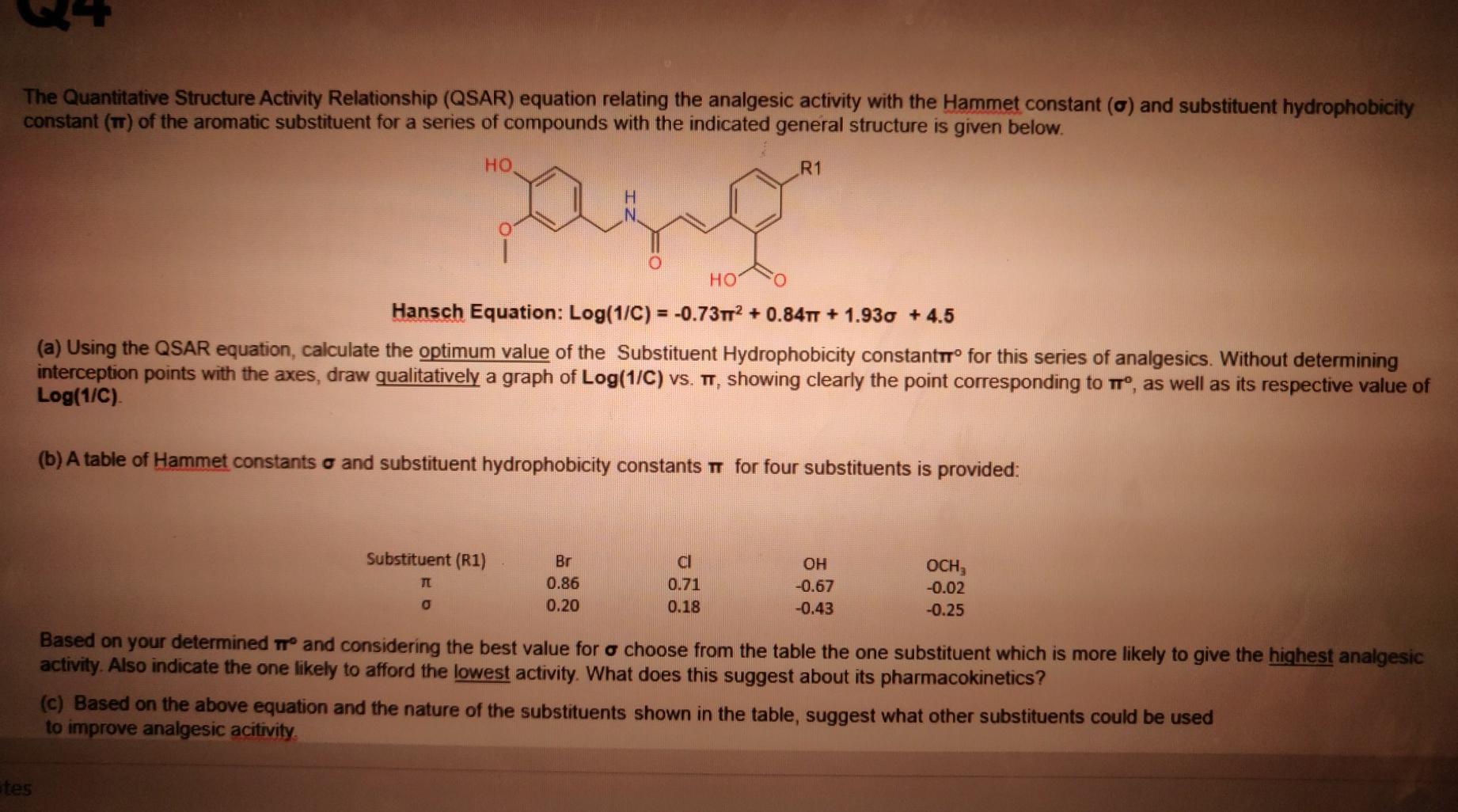
The Quantitative Structure Activity Relationship (QSAR) equation relating the analgesic activity with the Hammet constant (c) and substituent hydrophobicity constant (TT) of the aromatic substituent for a series of compounds with the indicated general structure is given below. . R1 HO Hansch Equation: Log(1/C) = -0.7312 + 0.84TT + 1.930 + 4.5 (a) Using the QSAR equation, calculate the optimum value of the Substituent Hydrophobicity constanttr for this series of analgesics. Without determining interception points with the axes, draw qualitatively a graph of Log(1/C) vs. TT, showing clearly the point corresponding to t, as well as its respective value of Log(1/C) (b) A table of Hammet constants o and substituent hydrophobicity constants o for four substituents is provided: Substituent (R1) Br 0.86 0.20 cl 0.71 0.18 OH -0.67 -0.43 OCH, -0.02 -0.25 0 Based on your determined and considering the best value for o choose from the table the one substituent which is more likely to give the highest analgesic activity. Also indicate the one likely to afford the lowest activity. What does this suggest about its pharmacokinetics? (c) Based on the above equation and the nature of the substituents shown in the table, suggest what other substituents could be used to improve analgesic acitivity. tes The Quantitative Structure Activity Relationship (QSAR) equation relating the analgesic activity with the Hammet constant (c) and substituent hydrophobicity constant (TT) of the aromatic substituent for a series of compounds with the indicated general structure is given below. . R1 HO Hansch Equation: Log(1/C) = -0.7312 + 0.84TT + 1.930 + 4.5 (a) Using the QSAR equation, calculate the optimum value of the Substituent Hydrophobicity constanttr for this series of analgesics. Without determining interception points with the axes, draw qualitatively a graph of Log(1/C) vs. TT, showing clearly the point corresponding to t, as well as its respective value of Log(1/C) (b) A table of Hammet constants o and substituent hydrophobicity constants o for four substituents is provided: Substituent (R1) Br 0.86 0.20 cl 0.71 0.18 OH -0.67 -0.43 OCH, -0.02 -0.25 0 Based on your determined and considering the best value for o choose from the table the one substituent which is more likely to give the highest analgesic activity. Also indicate the one likely to afford the lowest activity. What does this suggest about its pharmacokinetics? (c) Based on the above equation and the nature of the substituents shown in the table, suggest what other substituents could be used to improve analgesic acitivity. tes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


