Question: The reactant concentration in a zero-order reaction was 5.00102M after 200s and 4.00102M after 305s. What is the rate constant for this reaction? Express your
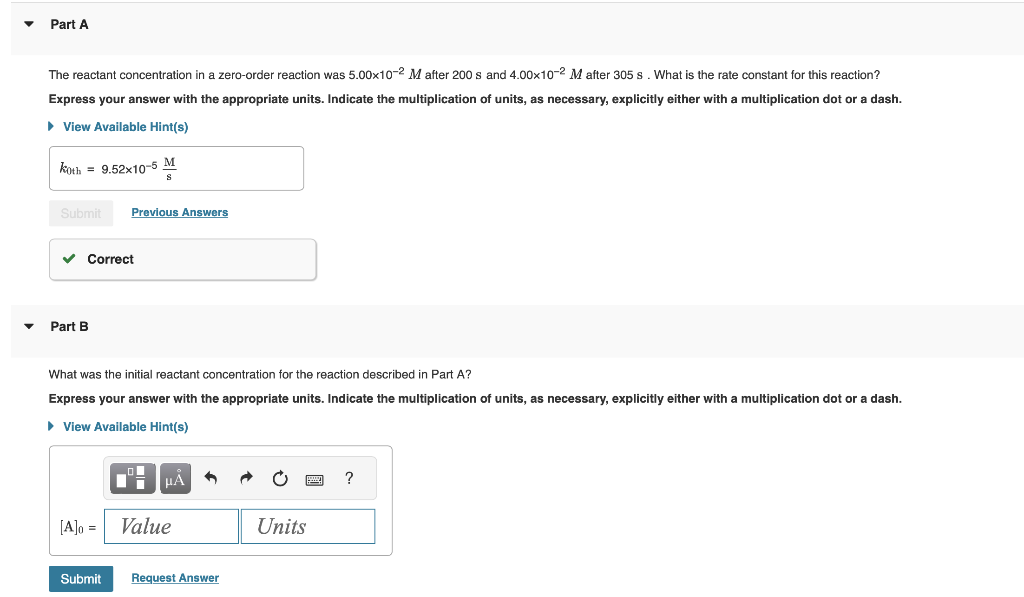
The reactant concentration in a zero-order reaction was 5.00102M after 200s and 4.00102M after 305s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. Part B What was the initial reactant concentration for the reaction described in Part A? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


