Question: please help The reactant concentration in a zero-order reaction was 9.00102molL1 after 115s and 1.00102molL1 after 370s. What is the rate constant for this reaction?
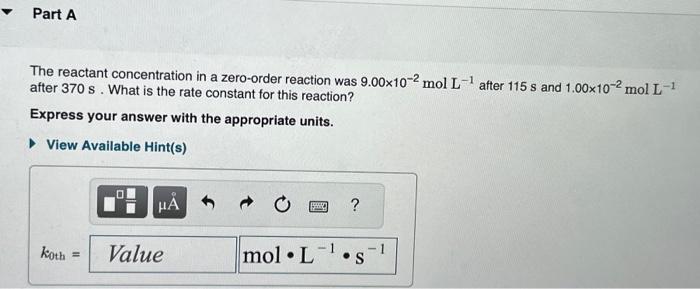
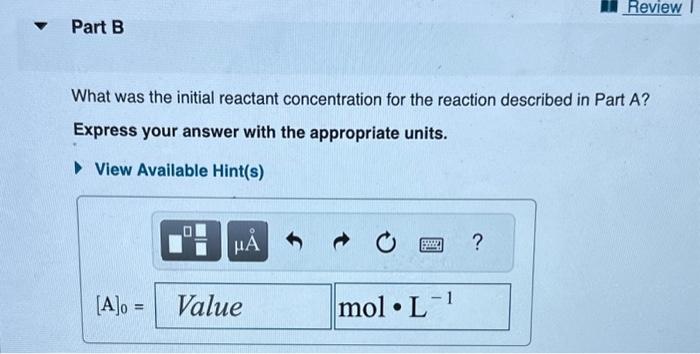
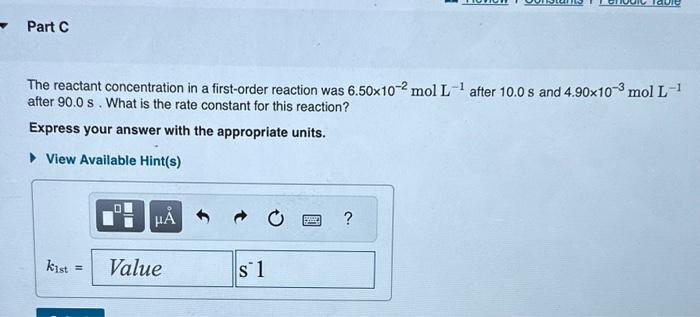
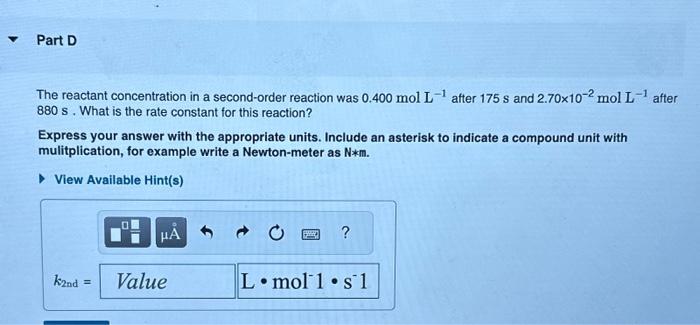
The reactant concentration in a zero-order reaction was 9.00102molL1 after 115s and 1.00102molL1 after 370s. What is the rate constant for this reaction? Express your answer with the appropriate units. What was the initial reactant concentration for the reaction described in Part A? Express your answer with the appropriate units. The reactant concentration in a first-order reaction was 6.50102molL1 after 10.0s and 4.90103molL1 after 90.0s. What is the rate constant for this reaction? Express your answer with the appropriate units. The reactant concentration in a second-order reaction was 0.400molL1 after 175s and 2.70102molL1 after 880s. What is the rate constant for this reaction? Express your answer with the appropriate units. Include an asterisk to indicate a compound unit with mulitplication, for example write a Newton-meter as Nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


