Question: The integrated rate laws for zero-, first-, and secondorder reaction may be arranged such that they resemble the equation for a straight line, y=mx+b The
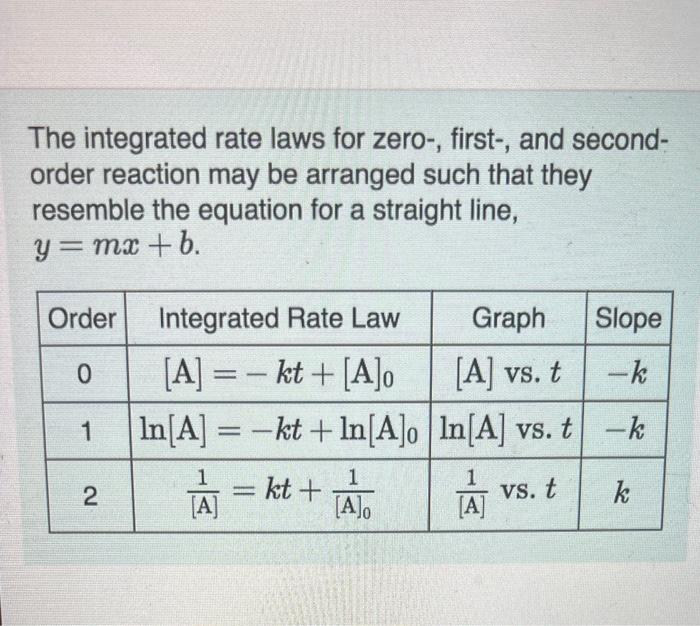
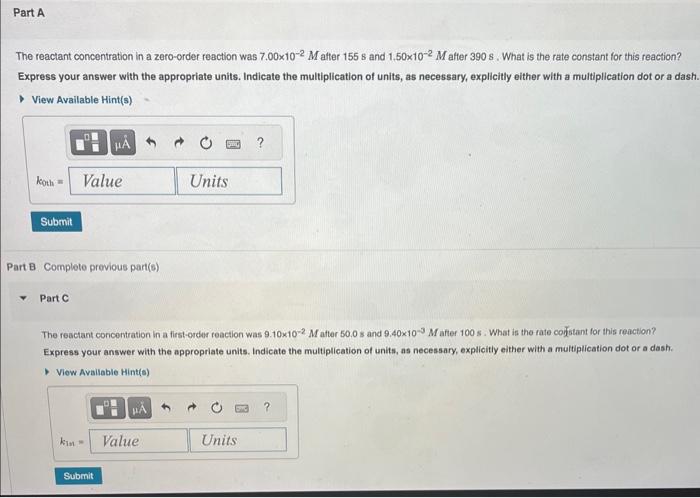
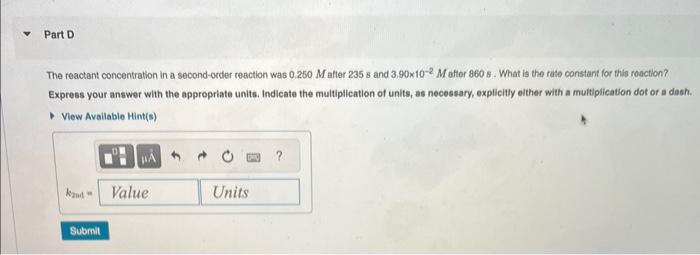
The integrated rate laws for zero-, first-, and secondorder reaction may be arranged such that they resemble the equation for a straight line, y=mx+b The reactant concentration in a zero-order reaction was 7.00102M after 155s and 1.50102M after 390s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. Part B Complote previous part(s) Part C The reactant concentration in a first-order teaction was 9.10102M atter 50.0s and 9.40103M after 100s. What is the rate corgstant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. The reactant concentration in a second-order reaction was 0.250M after 235s and 3,90102M afier 860s. What is the rate constant for this roaction? Express your answer wlth the appropriate units. Indicate the multipilcation of units, as necessary, explicitly either with a multiplication dot or a dash
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


