Question: The reaction A+B is conducted in a constant volume batch reactor. The following data of the concentration of A was obtained during the reaction. t
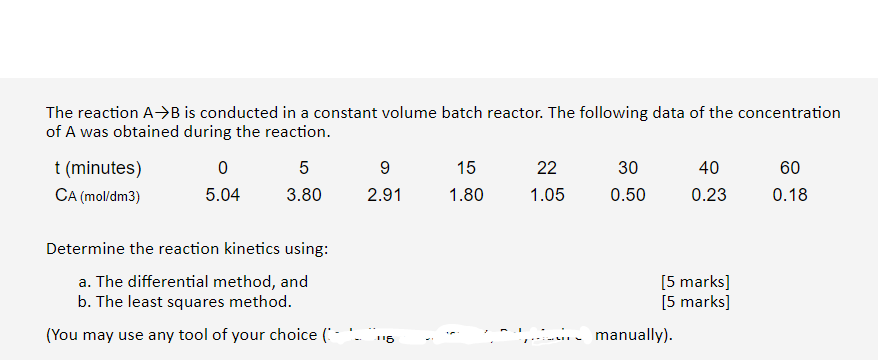
The reaction A+B is conducted in a constant volume batch reactor. The following data of the concentration of A was obtained during the reaction. t (minutes) 0 5 9 15 22 30 40 60 CA (mol/dm3) 5.04 3.80 2.91 1.80 1.05 0.50 0.23 0.18 Determine the reaction kinetics using: a. The differential method, and b. The least squares method. (You may use any tool of your choice ( (5 marks] [5 marks] 1 115 - manually)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


