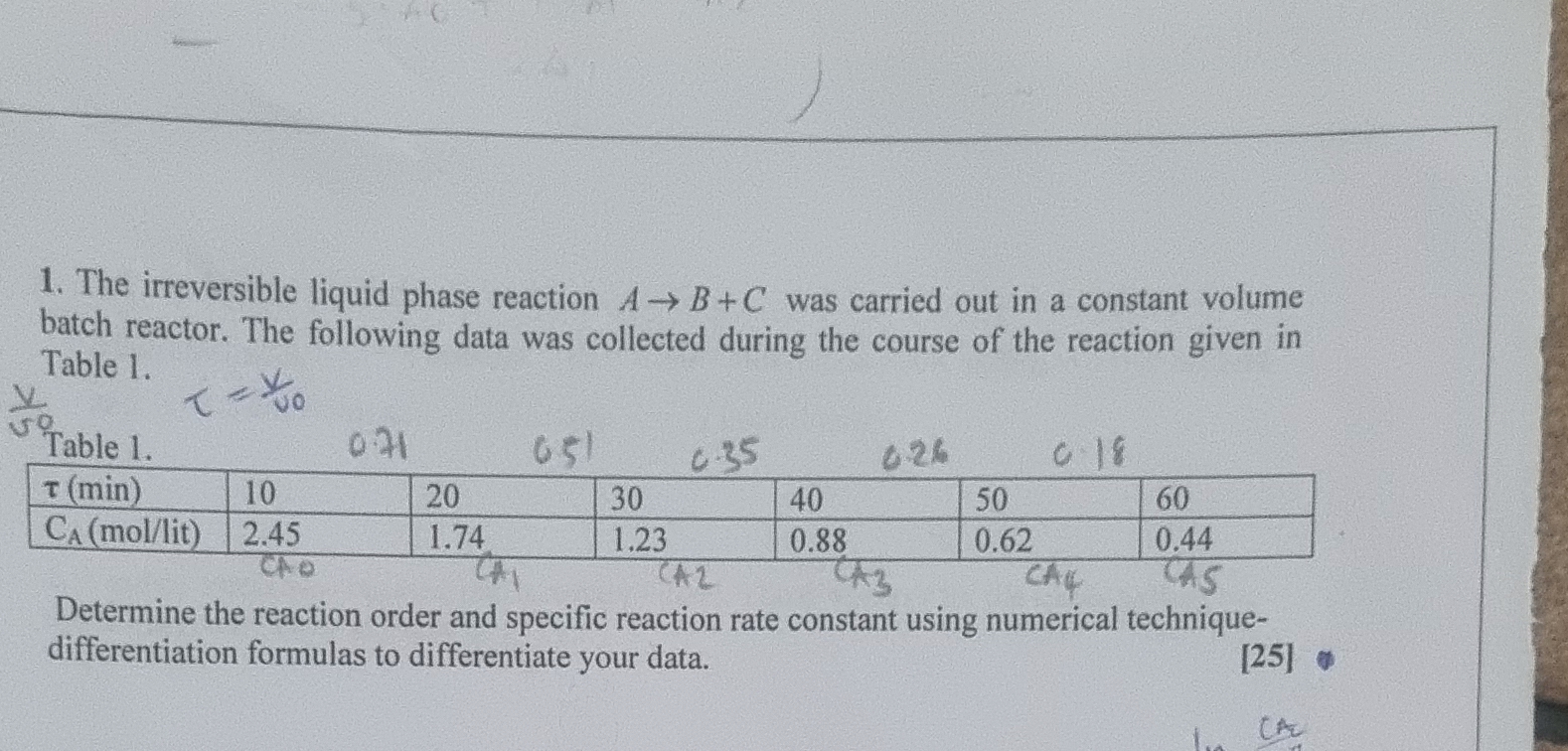
Question: The irreversible liquid phase reaction A B + C was carried out in a constant volume batch reactor. The following data was collected during the
The irreversible liquid phase reaction was carried out in a constant volume batch reactor. The following data was collected during the course of the reaction given in Table
Table
table
Determine the reaction order and specific reaction rate constant using numerical techniquedifferentiation formulas to differentiate your data.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


