Question: The reaction AB will take place in a continuous flow reactor. Calculate the CSTR volume (in dm) required to consume 5,8% of A, if the
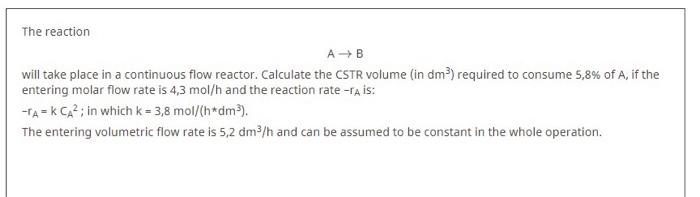
The reaction AB will take place in a continuous flow reactor. Calculate the CSTR volume (in dm) required to consume 5,8% of A, if the entering molar flow rate is 4,3 mol/h and the reaction rate -ra is: -TANK CAP: in which k = 3,8 mol/(hdm). The entering volumetric flow rate is 5,2 dm3/h and can be assumed to be constant in the whole operation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


