Question: The reaction occurring in the CSTR is a) b) AB, ra = -kCA The reactor is initially at steady state, with volumetric flow rate V
The reaction occurring in the CSTR is 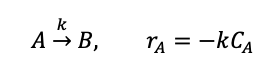
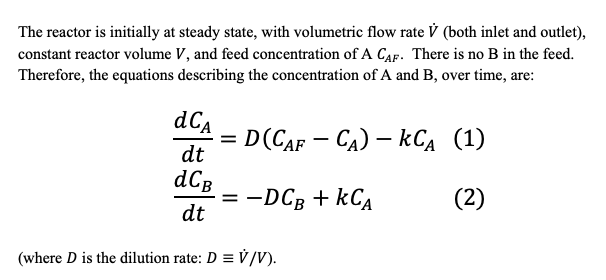
a)

b)
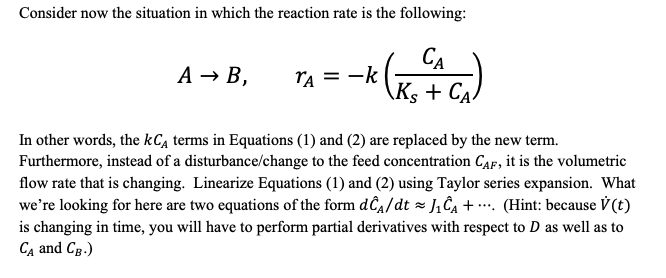
AB, ra = -kCA The reactor is initially at steady state, with volumetric flow rate V (both inlet and outlet), constant reactor volume V, and feed concentration of A Cap. There is no B in the feed. Therefore, the equations describing the concentration of A and B, over time, are: dCA dt = ED(CAF - Ca) kCA (1) E dCB =-DC8+ kCA (2) dt (where D is the dilution rate: D = V/V). = For time t
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


