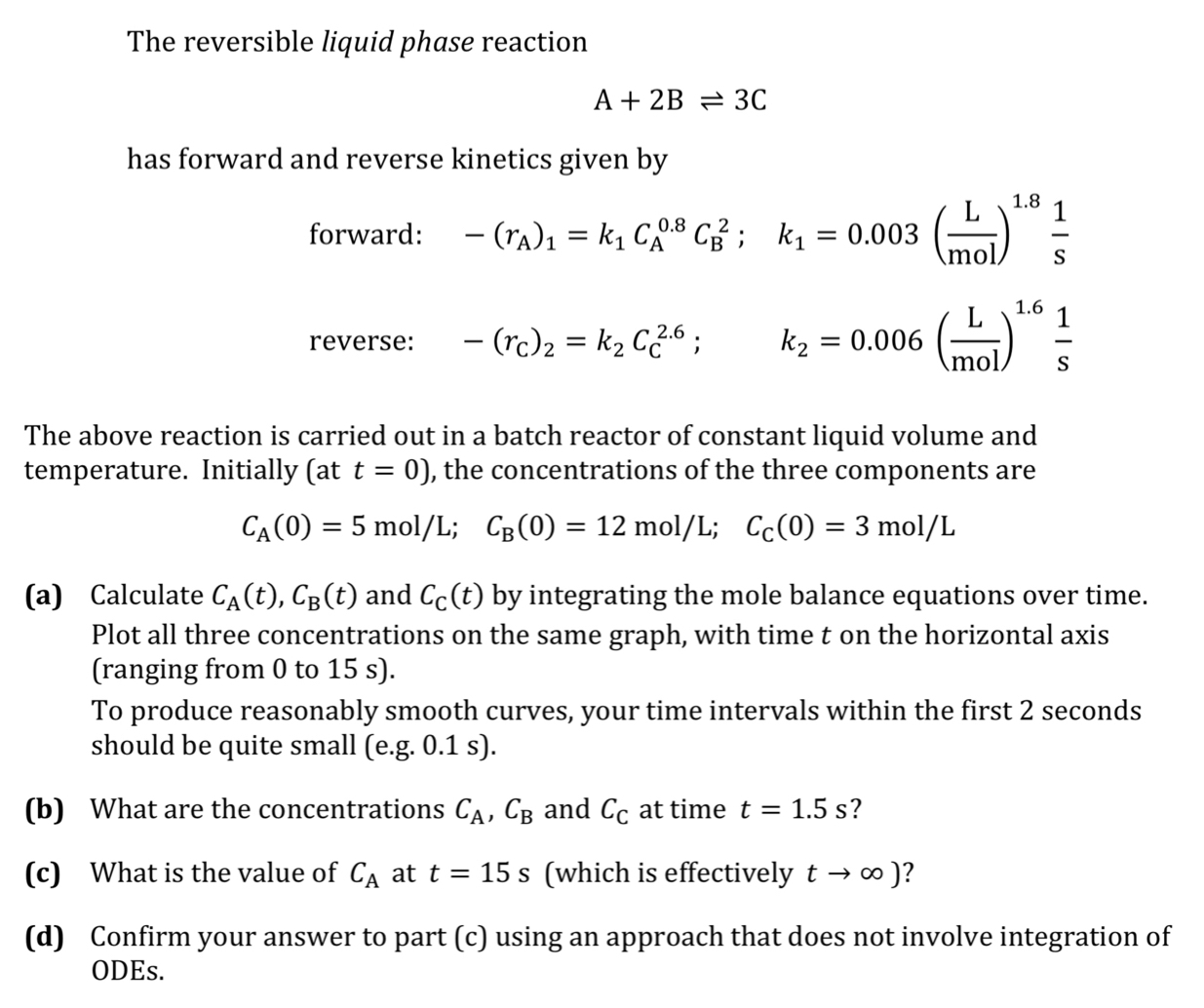
Question: The reversible liquid phase reaction A + 2 B 3 C has forward and reverse kinetics given by forward: - ( r A ) 1
The reversible liquid phase reaction
has forward and reverse kinetics given by
forward: ;
reverse: ;
The above reaction is carried out in a batch reactor of constant liquid volume and temperature. Initially at the concentrations of the three components are
;;
a Calculate and by integrating the mole balance equations over time. Plot all three concentrations on the same graph, with time on the horizontal axis ranging from to
To produce reasonably smooth curves, your time intervals within the first seconds should be quite small eg
b What are the concentrations and at time
c What is the value of at which is effectively
d Confirm your answer to part c using an approach that does not involve integration of ODEs.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


