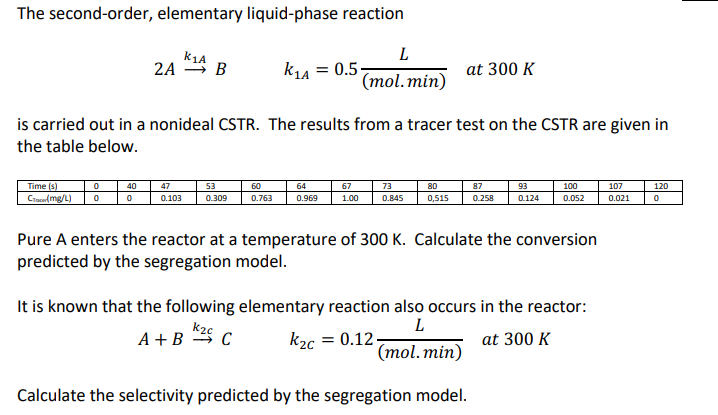
Question: The second - order, elementary liquid - phase reaction 2 A k 1 A B , k 1 A = 0 . 5 L (
The secondorder, elementary liquidphase reaction
is carried out in a nonideal CSTR The results from a tracer test on the CSTR are given in
the table below.
Pure A enters the reactor at a temperature of Calculate the conversion
predicted by the segregation model.
It is known that the following elementary reaction also occurs in the reactor:
Calculate the selectivity predicted by the segregation model.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


