Question: The spin-orbit interaction can cause a spectral line to split into two lines. A good example of this is the 4P state in sodium which

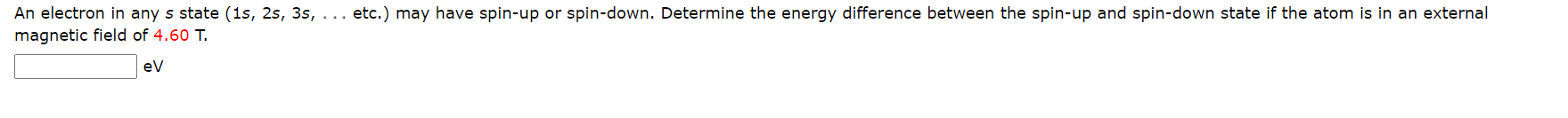


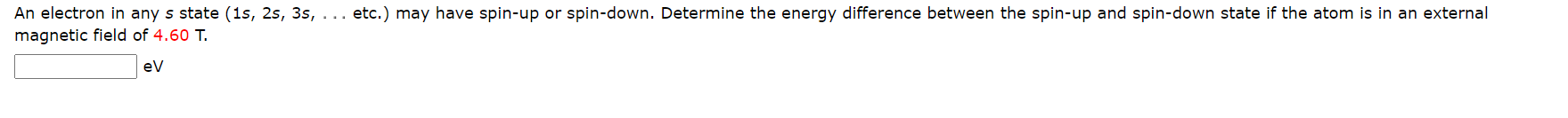

The spin-orbit interaction can cause a spectral line to split into two lines. A good example of this is the 4P state in sodium which is split into the 4451/2 and 4P3/2 state with the respective wavelengths associated with a transition to the ground state being A = 330.30 nm and A = 330.24 nm. Determine the magnitude of the internal magnetic field (in T) causing this splitting. :T An electron in any s state (1s, 2s, 3s, . .. etc.) may have spin-up or spin-down. Determine the energy difference between the spin-up and spin-down state if the atom is in an external magnetic field of 4.60 T. evThe spin-orbit interaction can cause a spectral line to split into two lines. A good example of this is the 4P state in sodium which is split into the 4P1/2 and 4P3;2 state with the respective wavelengths associated with a transition to the ground state being A = 330.30 nm and = 330.24 nm. Determine the magnitude of the internal magnetic field (in T) causing this splitting. x ' Can you determine the energy difference between the two states from the wavelengths of the photons emitted for the two different transitions to the ground state? Can you find or develop an expression for this energy difference as a function of the internal magnetic field? See if you can combine this information to obtain the magnitude of the internal magnetic field. T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


