Question: The unsaturated ester at left can be reduced using lithium and ammonia, and the resulting ester enolate can be alkylated with methyl iodide to give
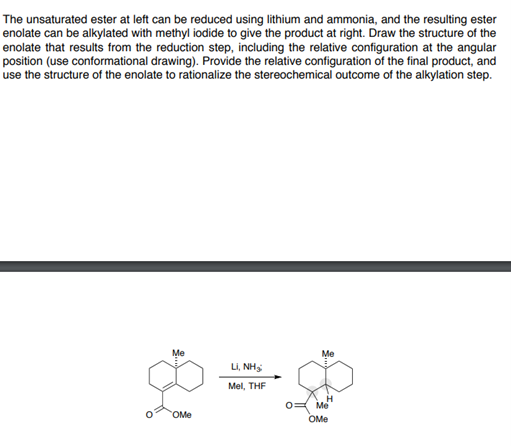
The unsaturated ester at left can be reduced using lithium and ammonia, and the resulting ester enolate can be alkylated with methyl iodide to give the product at right. Draw the structure of the enolate that results from the reduction step, including the relative configuration at the angular position (use conformational drawing). Provide the relative configuration of the final product, and use the structure of the enolate to rationalize the stereochemical outcome of the alkylation step. Me Me L, NH3 Mel, THF OM Me OME
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


