Question: The van't Hoff factor, i, is equal to i=thecalculatedmolesofsoluteinsolutionthecalculatedmolesofsoluteinsolution2 i= the calculated moles of solute in solution i= the observed moles of particles in solution
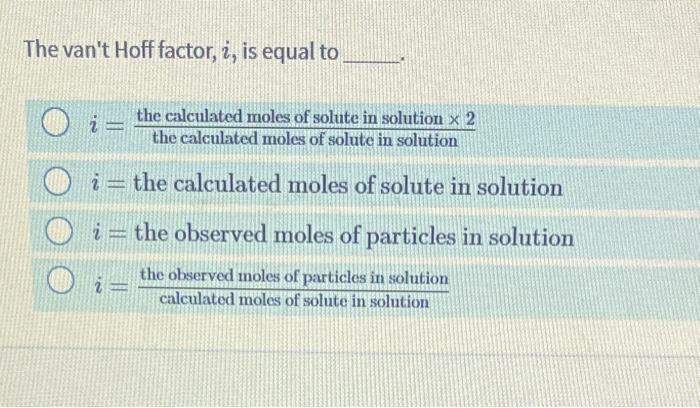
The van't Hoff factor, i, is equal to i=thecalculatedmolesofsoluteinsolutionthecalculatedmolesofsoluteinsolution2 i= the calculated moles of solute in solution i= the observed moles of particles in solution i=calculatedmolesofsoluteinsolutionthcobservedmolesofparticlesinsolution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


