Question: Colligative properties, such as boiling point elevation, depend on the number of dissolved particles in solution. For nonelectrolytes, no dissociation occurs, and so you can
Colligative properties, such as boiling point elevation,
depend on the number of dissolved particles in
solution. For nonelectrolytes, no dissociation occurs,
and so you can use the number of moles of solute to
calculate both molality and molarity. In contrast,
electrolytes dissociate, and therefore the molality and
molarity must be calculated based on the number of
moles of dissociated particles or ions.
There are two ions per formula unit of NaCl.
Therefore, we would expect the freezingpoint
depression of a NaCl solution to be twice that
of a sugar solution of the same concentration.
However, it has been experimentally determined that
in the typical unsaturated solution for the salt
solution is only times that of the sugar solution.
This indicates that not all ion pairs in the NaCl
solution are dissociated. The number is called the
van't Hoff factor symbolized by and can be thought
of as the number of dissociated particles per NaCl
formula unit. This factor changes based on the
concentration of the solution, and each salt will have a
series of experimentally determined values.
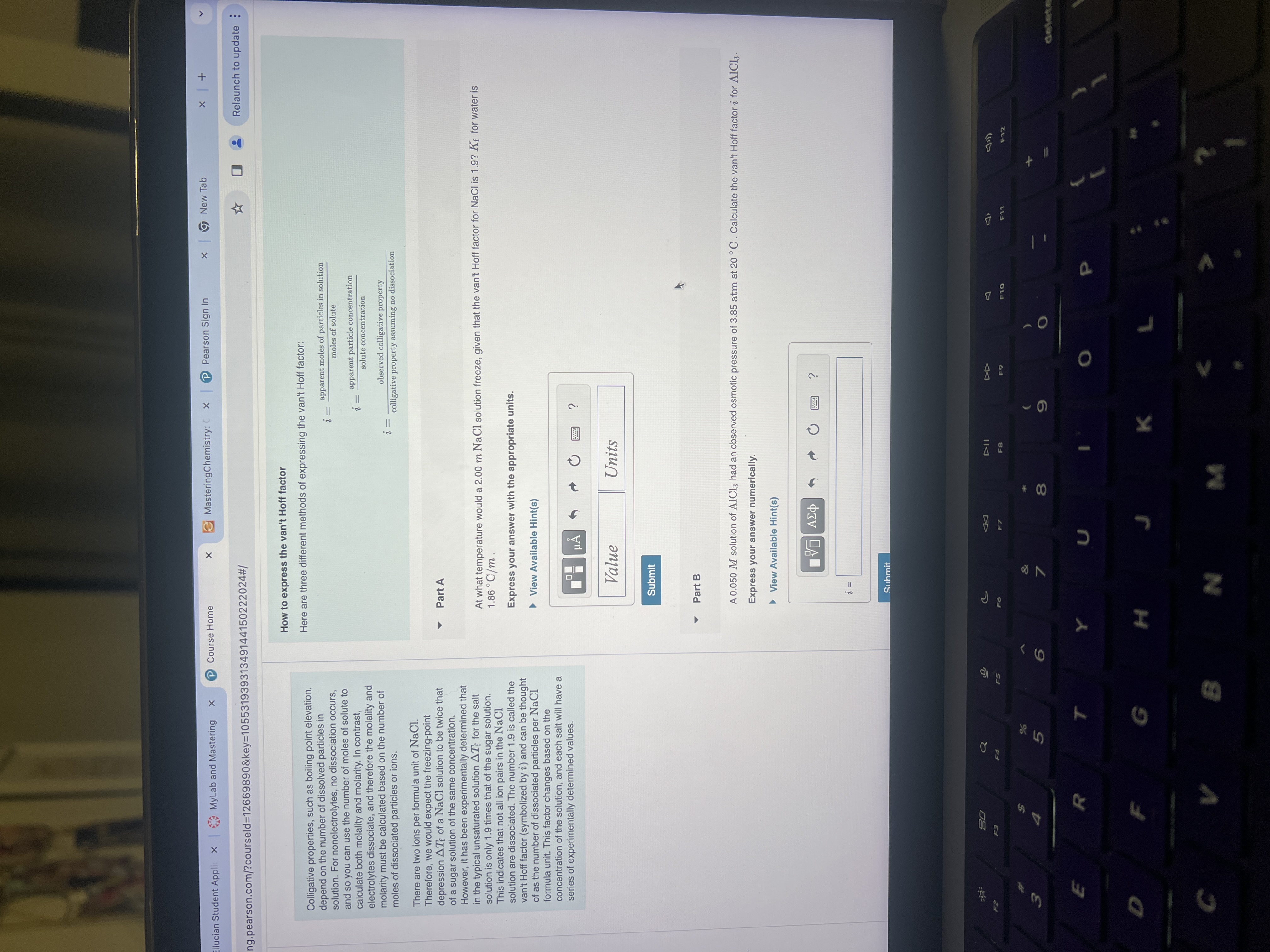
How to express the van't Hoff factor
Here are three different methods of expressing the van't Hoff factor:
Part A
At what temperature would a mNaCl solution freeze, given that the van't Hoff factor for NaCl is for water is
Express your answer with the appropriate units.
View Available Hints
Part B
A solution of had an observed osmotic pressure of atm at Calculate the van't Hoff factor i for
Express your answer numerically.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


