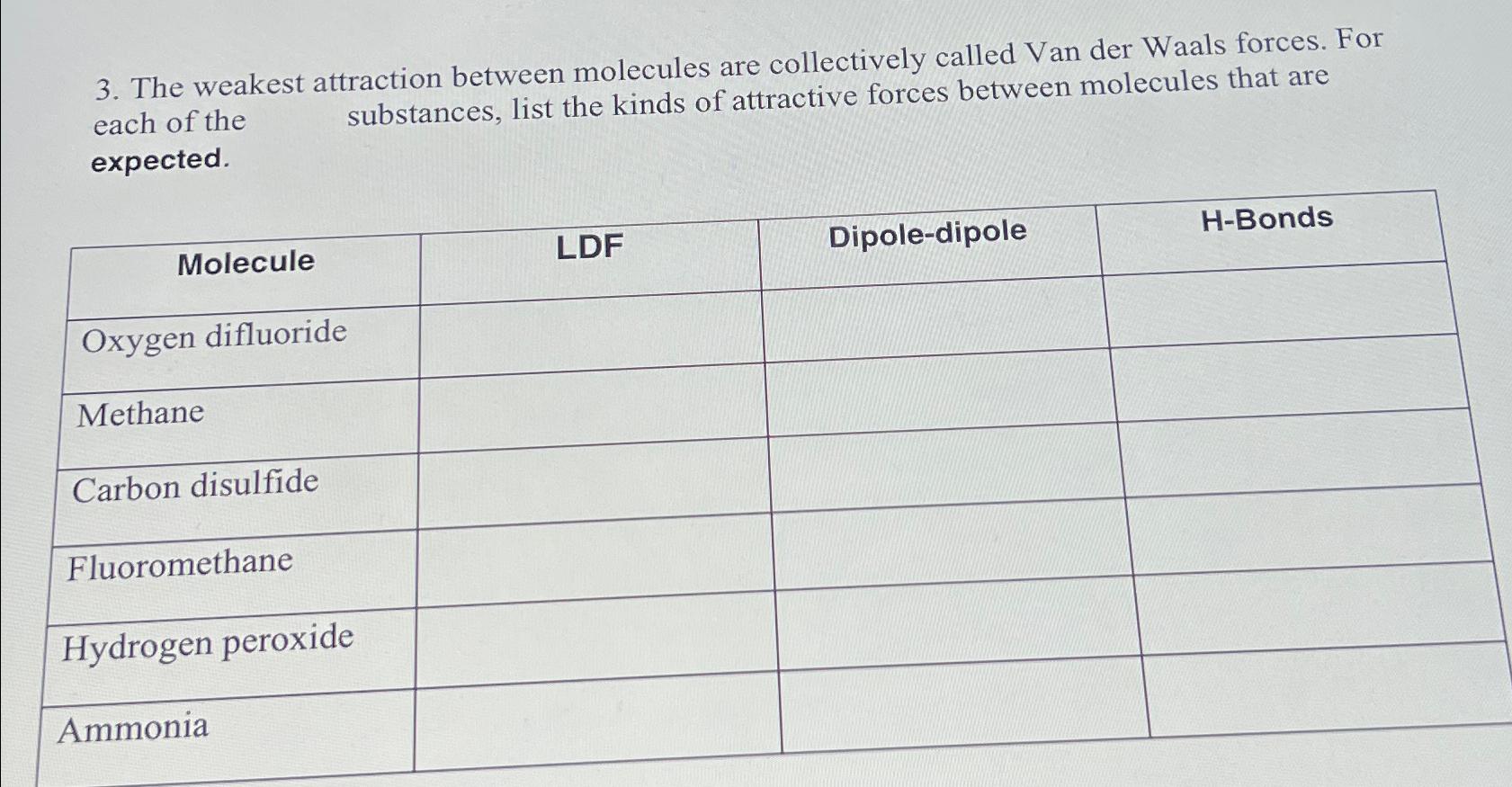
Question: The weakest attraction between molecules are collectively called Van der Waals forces. For each of the substances, list the kinds of attractive forces between molecules
The weakest attraction between molecules are collectively called Van der Waals forces. For each of the substances, list the kinds of attractive forces between molecules that are expected.
tableMoleculeLDFDipoledipole,HBondsOxygen difluoride,,,MethaneCarbon disulfide,,drogen peroxide,,,Ammonia
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


