Question: There are three containers each containing a gas. Box A contains 1 mole of some unknown gas at 100C. Box B contains some unknown amount
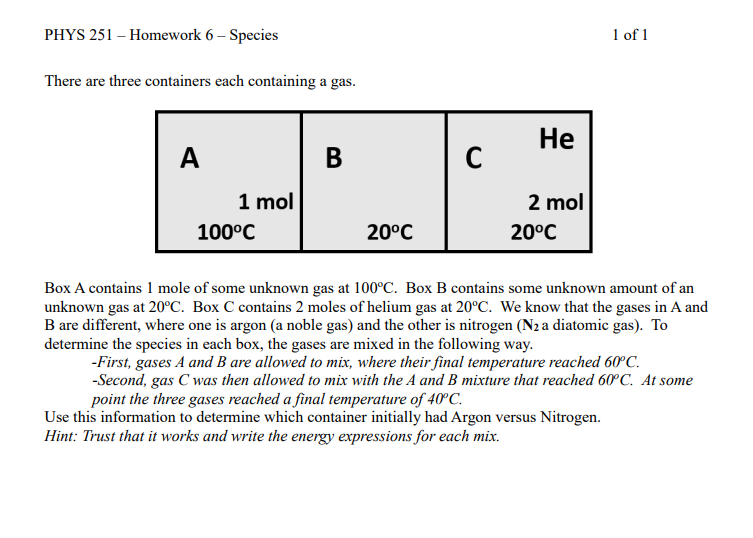
There are three containers each containing a gas. Box A contains 1 mole of some unknown gas at 100C. Box B contains some unknown amount of an unknown gas at 20C. Box C contains 2 moles of helium gas at 20C. We know that the gases in A and B are different, where one is argon (a noble gas) and the other is nitrogen ( N2 a diatomic gas). To determine the species in each box, the gases are mixed in the following way. -First, gases A and B are allowed to mix, where their final temperature reached 60C. -Second, gas C was then allowed to mix with the A and B mixture that reached 60C. At some point the three gases reached a final temperature of 40C. Use this information to determine which container initially had Argon versus Nitrogen. Hint: Trust that it works and write the energy expressions for each mix
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


