Question: There are two trials for each analysis. Do the calculations for each trial separately then average the data. Gravimetric Analysis Gravimetric Analysis Mass of analyte
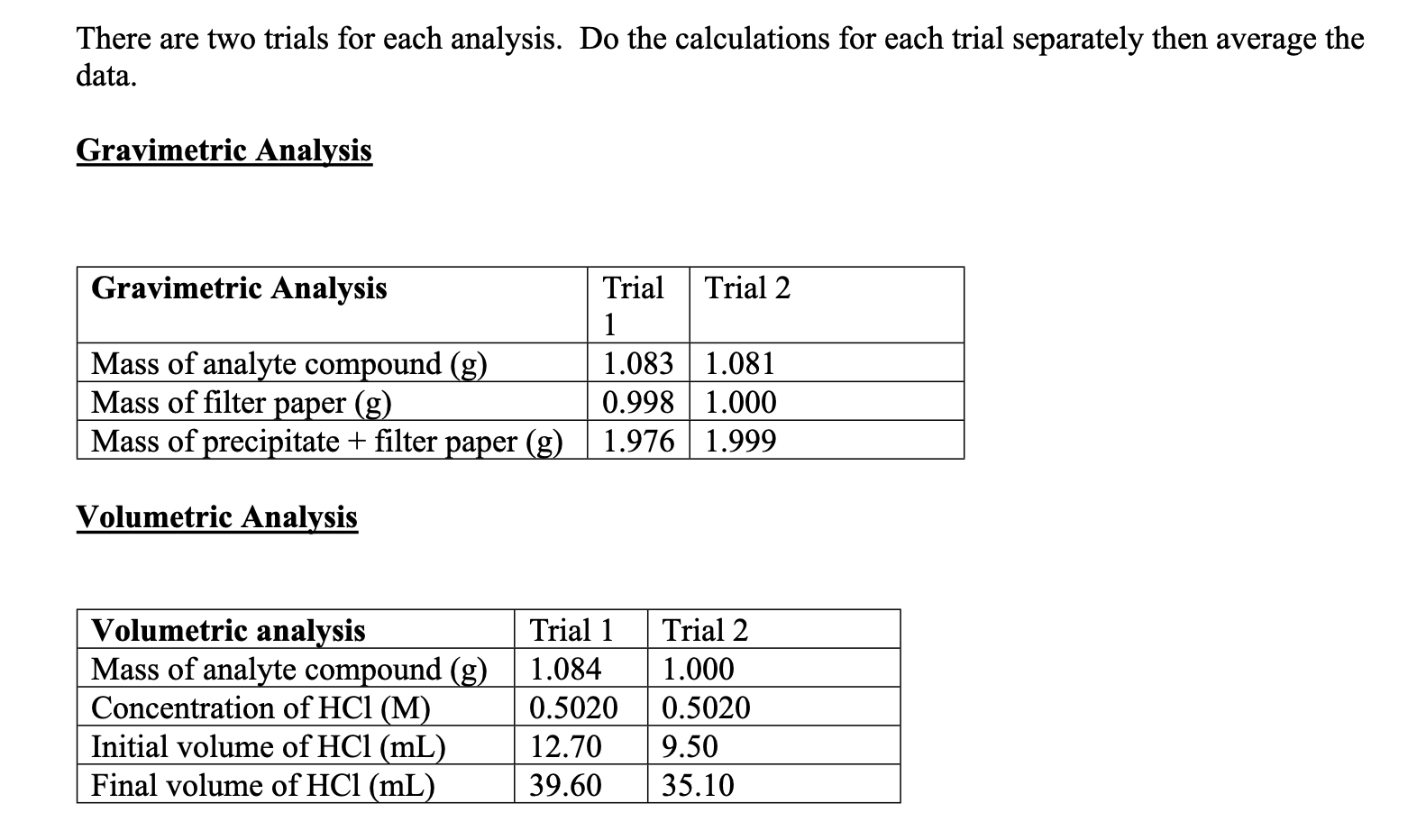
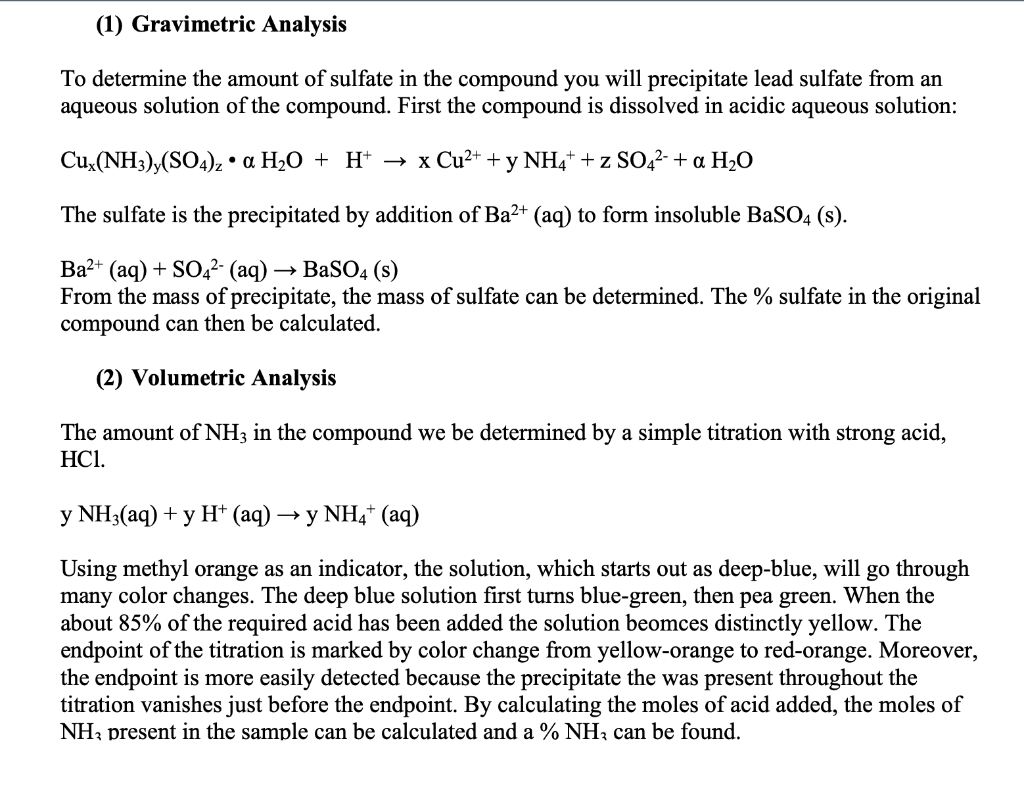
There are two trials for each analysis. Do the calculations for each trial separately then average the data. Gravimetric Analysis Gravimetric Analysis Mass of analyte compound (g) Mass of filter paper (g) Mass of precipitate + filter paper (g) Trial Trial 2 1 1.083 1.081 0.998 1.000 1.976 1.999 Volumetric Analysis Volumetric analysis Mass of analyte compound (g) Concentration of HCI (M) Initial volume of HCl (mL) Final volume of HCl (mL) Trial 1 1.084 0.5020 12.70 39.60 Trial 2 1.000 0.5020 9.50 35.10 (1) Gravimetric Analysis To determine the amount of sulfate in the compound you will precipitate lead sulfate from an aqueous solution of the compound. First the compound is dissolved in acidic aqueous solution: Cux(NH3),(SO4)2 a H2O + H+ + x Cu2+ + y NH4+ + z SO42- + a H2O The sulfate is the precipitated by addition of Ba2+ (aq) to form insoluble BaSO4(s). Ba2+ (aq) + SO42- (aq) BaSO4 (s) From the mass of precipitate, the mass of sulfate can be determined. The % sulfate in the original compound can then be calculated. (2) Volumetric Analysis The amount of NH3 in the compound we be determined by a simple titration with strong acid, HCl. y NH3(aq) + y H+ (aq) y NH4+ (aq) Using methyl orange as an indicator, the solution, which starts out as deep-blue, will go through many color changes. The deep blue solution first turns blue-green, then pea green. When the about 85% of the required acid has been added the solution beomces distinctly yellow. The endpoint of the titration is marked by color change from yellow-orange to red-orange. Moreover, the endpoint is more easily detected because the precipitate the was present throughout the titration vanishes just before the endpoint. By calculating the moles of acid added, the moles of NHz present in the sample can be calculated and a % NHz can be found
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


