Question: these have to do with enthalpy can u pls help me answer the questions? if u can only answer 4 pls include question 11 as
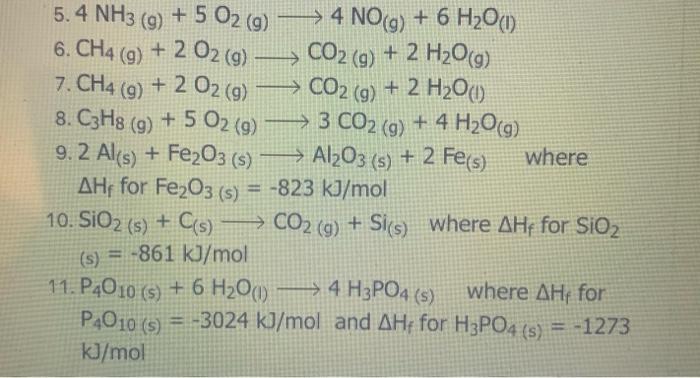
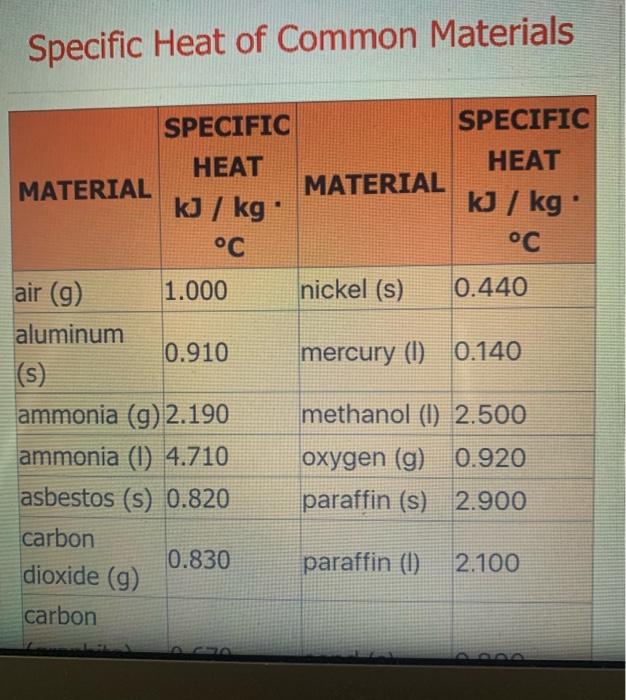
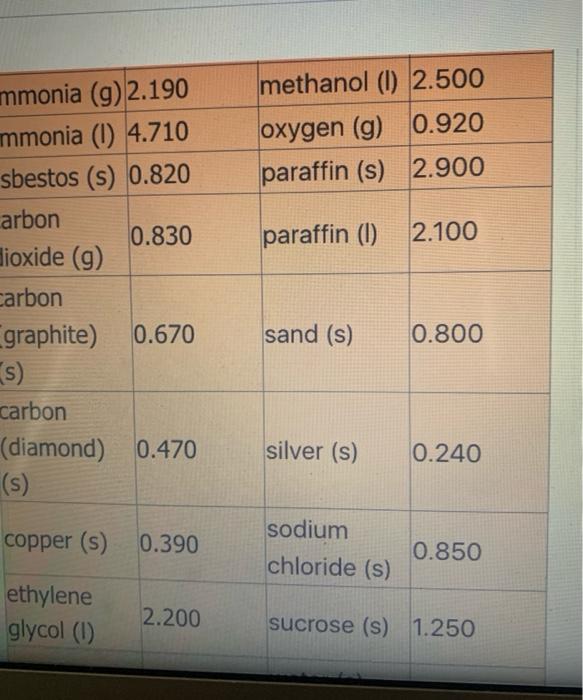
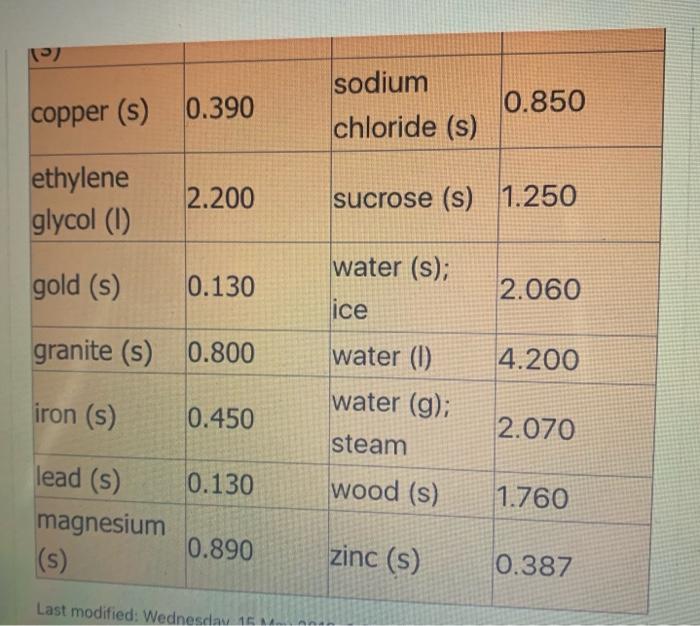
( 02 ) (9 5.4 NH3 (9) + 5 O2 (9) > 4 NO(g) + 6 H2O(1) 6. CH4 (g) + 2O2(g) > CO2 (9) + 2 H209) 7. CH4 (g) + 2O2 (g) CO2 (g) + 2 H200) 8. C3H8 (g) + 5 O2 (9) 9 + 5 O2 (9) > 3 CO2 (g) + 4 H2O(g) 9.2 Al(s) + Fe2O3(s) Al2O3 (s) + 2 Fe(s) where AHF for Fe2O3 (s) = -823 kJ/mol 10. SiO2 (s) + C(s) CO2 (g) + Si(s) where AHF for SiO2 (s) = -861 kJ/mol ( 11. P4010 (s) + 6 H2001) > 4 H3PO4 (s) where AHF for P4010 (s) = -3024 kJ/mol and AH for H3PO4 (s) = -1273 kJ/mol -> Specific Heat of Common Materials SPECIFIC SPECIFIC HEAT HEAT MATERIAL MATERIAL kJ / kg kJ / kg C C nickel (s) 0.440 mercury (0) 0.140 1 air (9) 1.000 aluminum 0.910 (s) ammonia (9) 2.190 ammonia (0) 4.710 asbestos (s) 0.820 carbon 0.830 dioxide (g) carbon methanol (1) 2.500 oxygen (g) 0.920 paraffin (s) 2.900 paraffin (1) 2.100 methanol (1) 2.500 oxygen (g) 0.920 paraffin (s) 2.900 paraffin (1) 2.100 mmonia (g) 2.190 mmonia (1) 4.710 sbestos (s) 0.820 arbon 0.830 dioxide (9) carbon graphite) 0.670 s) carbon (diamond) 0.470 (s) sand (s) 0.800 silver (s) 0.240 copper (s) 0.390 sodium chloride (s) 0.850 ethylene glycol (1) 2.200 sucrose (s) 1.250 sodium chloride (s) 0.850 copper (s) 0.390 ethylene glycol (1) 2.200 sucrose (s) 1.250 water (s); gold (s) 0.130 2.060 ice granite (s) 0.800 4.200 iron (s) water (1) water (g); steam 0.450 2.070 0.130 wood (s) 1.760 lead (s) magnesium (s) 0.890 zinc (s) 0.387 Last modified: Wednesday 16
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


