Question: This compound is a liquid (boiling point 7172C ) that can be deprotonated by solium amide, a very strong buse. The Mass, IR. 'H NMR-,
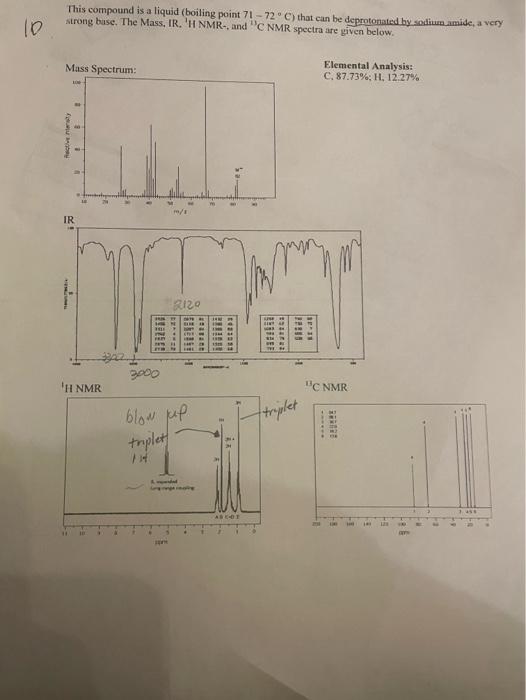


This compound is a liquid (boiling point 7172C ) that can be deprotonated by solium amide, a very strong buse. The Mass, IR. 'H NMR-, and "C NMR spectra are given below. Elemental Analysis: C. 87.73%,H,12,27% ORGANIC CHEMIStRy PRE-LAB AND DATA REPORT SPECTROSCOPY Name: Date: Lab Section: Locker: Unknown number: Elemental Analysis: \% C: Empirical Formula calculation: Empirical Formula: Molar Mass from Mass Spectrum: 6/mel Molecular Formula calculation: Molecular Formula: Elements of Unsaturation: /100Total IR Spectrum data: From the IR data, what is the (are) possible class(es) of your compound? 1 H NMR data: Number of unique signals: 13 C NMR data: Number of unique signals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


