Question: this curve. Please answer the following theme cheresting questions, a) What is the total energy required to raise the temperature of 135g of water from
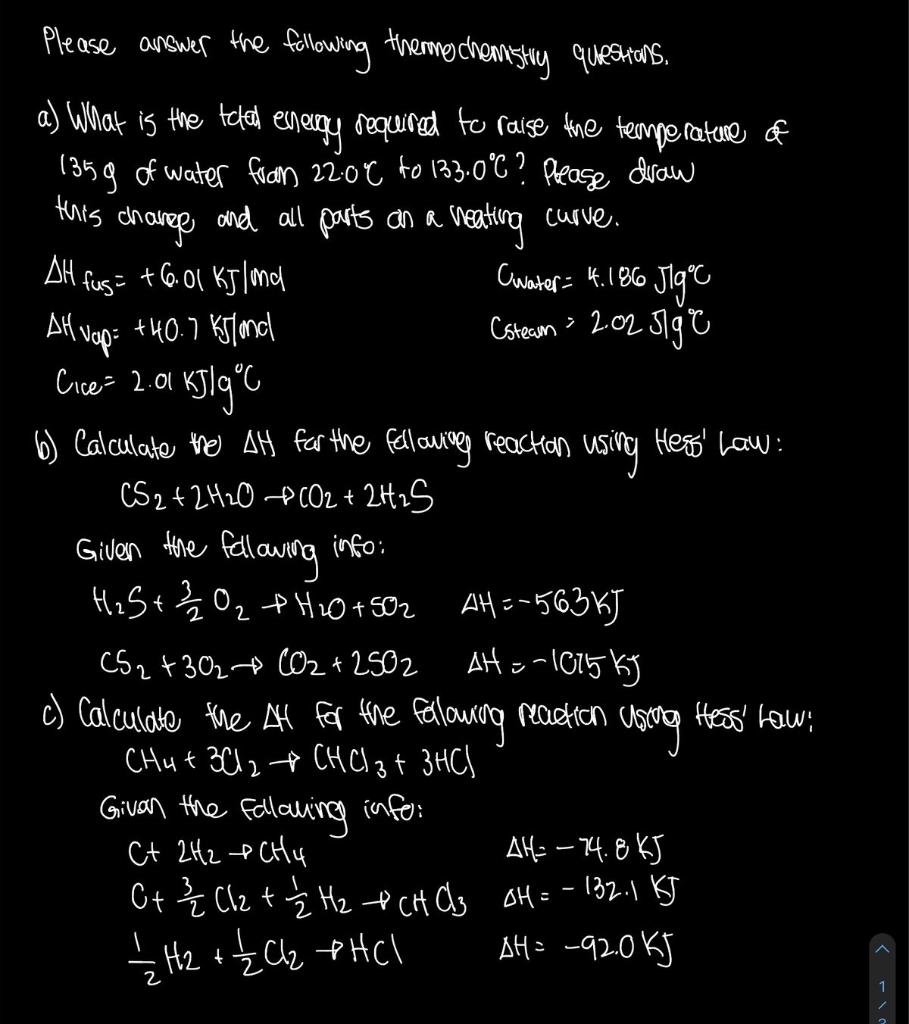
this curve. Please answer the following theme cheresting questions, a) What is the total energy required to raise the temperature of 135g of water from 22:00 to 133.0C? Please draw change and all parts an al neating DH fus : +6.01 kJlond (water = 4.186 36 Jig" AH Nap: +40.7 Khland Csteam = 2.02 Jigre Cice=2.01 kJlg'c b) Calculate the AH for the following reaction using thess' Law: CS2 + 2HO + (O2 + 2H2S Given the fellowing info: H2S+22 + H2O +502 AH = -563 kJ CS2+302 CO2 + 2502 AH = -1085 kg c) Calculate the All for the fallowing reaction using Hess' how CH4 + 3012 + CHCl3 + 3HCl Given the following infor: C+ 2H2 + CH4 AH=-74.8KJ Ct3 { Cl2 + 2 Hz & CHCl3 OH = - 132.1 KJ 1/2 H2 + { Cz PHCl AH= -92.0 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


