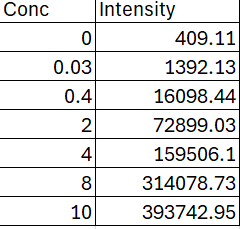
Question: This data was plot on a linear regression graph. Conc = concentration of Zinc. If you plot the intensity ( Y axis ) into the
This data was plot on a linear regression graph. Conc concentration of Zinc. If you plot the intensity Y axis into the equation y m X C for a capsule intensity and tablet intensity You should get concentration of Zinc in the capsule and tablet. Then to find the mass of the zinc, Concentration Mole Volume
Mole massRAM
Relative atomic mass multiply by Concentration give you the mass of Zinc. With this in mind what would the mass of zinc be in the tablet and capsule? Would this be the correct way of calculating it if not how? expert help needed please
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


