Question: This is a hw question so I changed the numbers used, I just want to learn the process of how to go about answering this.
 This is a hw question so I changed the numbers used, I just want to learn the process of how to go about answering this. Thank you in advance!
This is a hw question so I changed the numbers used, I just want to learn the process of how to go about answering this. Thank you in advance!
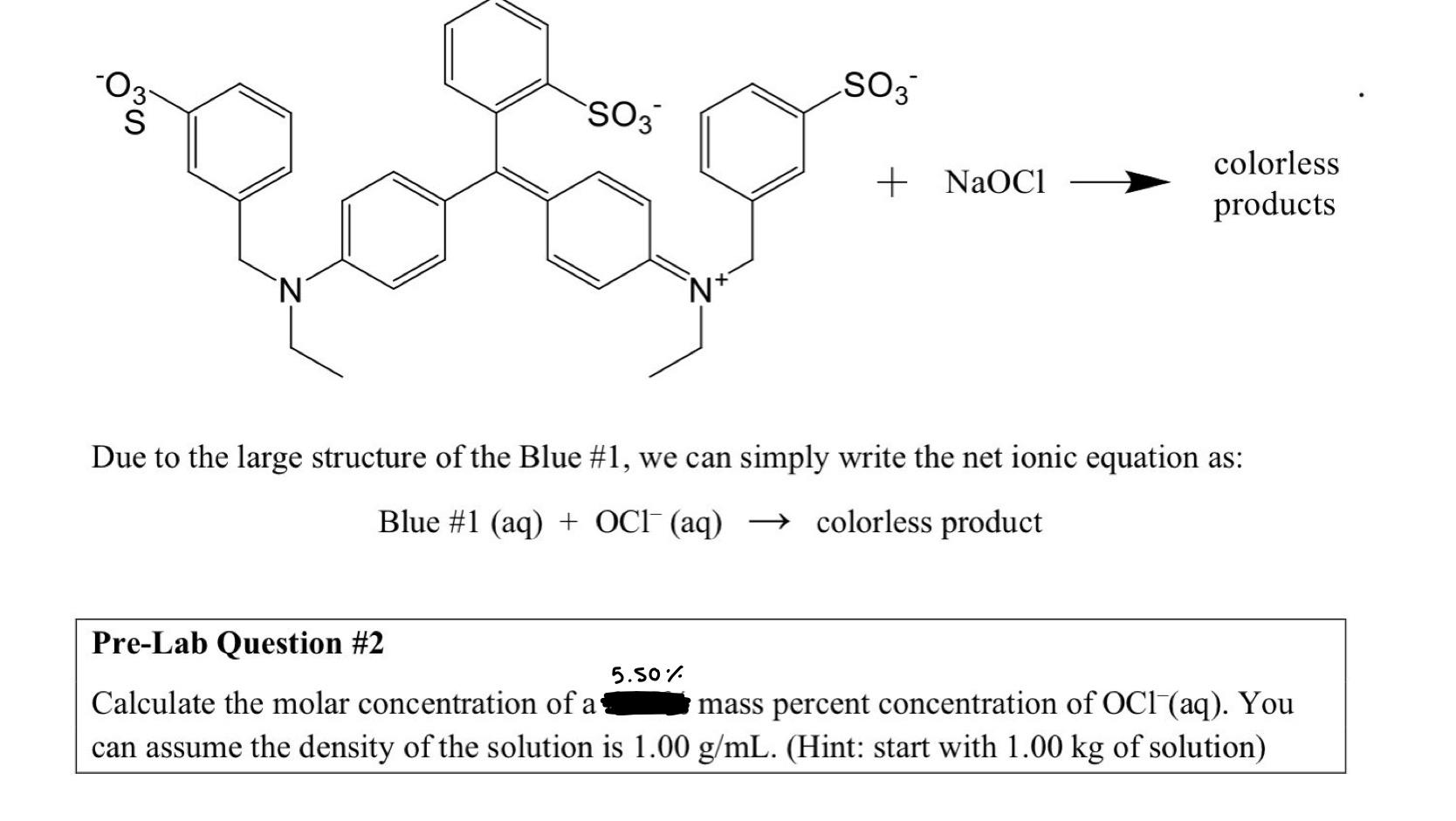
oos SO3 SO3 + NaOC1 colorless products 'N N+ Due to the large structure of the Blue #1, we can simply write the net ionic equation as: Blue #1 (aq) + OCI+ (aq) colorless product Pre-Lab Question #2 5.50 Calculate the molar concentration of a mass percent concentration of OCl(aq). You can assume the density of the solution is 1.00 g/mL. (Hint: start with 1.00 kg of solution)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


