Question: This is the question. All data is provided. i need neat and clean solution of this answer on a paper. Do full answer. All steps
This is the question. All data is provided. i need neat and clean solution of this answer on a paper. Do full answer. All steps should be performed and maon thing match all your answers before submitting. I will give u best feedback and likes from my friends as well.
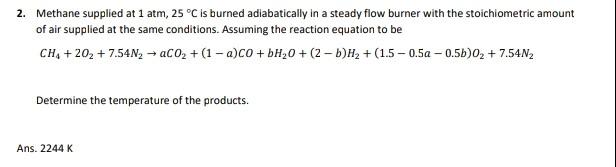
2. Methane supplied at 1 atm, 25 C is burned adiabatically in a steady flow burner with the stoichiometric amount of air supplied at the same conditions. Assuming the reaction equation to be CH4 +202 + 7.54N, - CO2 + (1 -a)co+bH,0 +(2-b)H2 + (1.5 -0.50 -0.5b)02 + 7.54N2 Determine the temperature of the products. Ans. 2244 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


