Question: This problem covers adiabatic operation of a CSTR with a reversible reaction. AB The reaction is reversible and the rate is given by: K(CA -
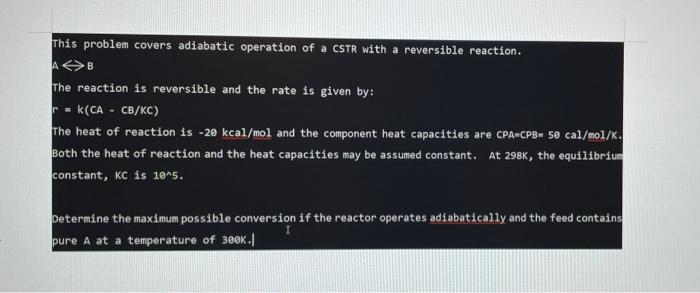
This problem covers adiabatic operation of a CSTR with a reversible reaction. AB The reaction is reversible and the rate is given by: K(CA - CB/KC) The heat of reaction is -20 kcal/mol and the component heat capacities are CPA-CPB- se cal/mol/K. Both the heat of reaction and the heat capacities may be assumed constant. At 2986, the equilibrium constant, kc is 10^5. r Determine the maximum possible conversion if the reactor operates adiabatically and the feed contains pure A at a temperature of 300K./
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


