Question: This question deals with four different titrations. A buffered solution was made: 0.5 M hydrochloric acid, 0.5 M sodium hydroxide, 1.0 M acetic acid solution,
This question deals with four different titrations. A buffered solution was made: 0.5 M hydrochloric acid, 0.5 M sodium hydroxide, 1.0 M acetic acid solution, and 0.149 g sodium acetate with a pH of 4.0
titrations used:
1) 1.0 M acetic acid with 0.5 M NaOH
2) the buffered solution with 0.5 M NaOH
3) 1.0 M acetic acid with 0.5 M HCL
4) the buffered solution with 0.5 M HCL
The Questions: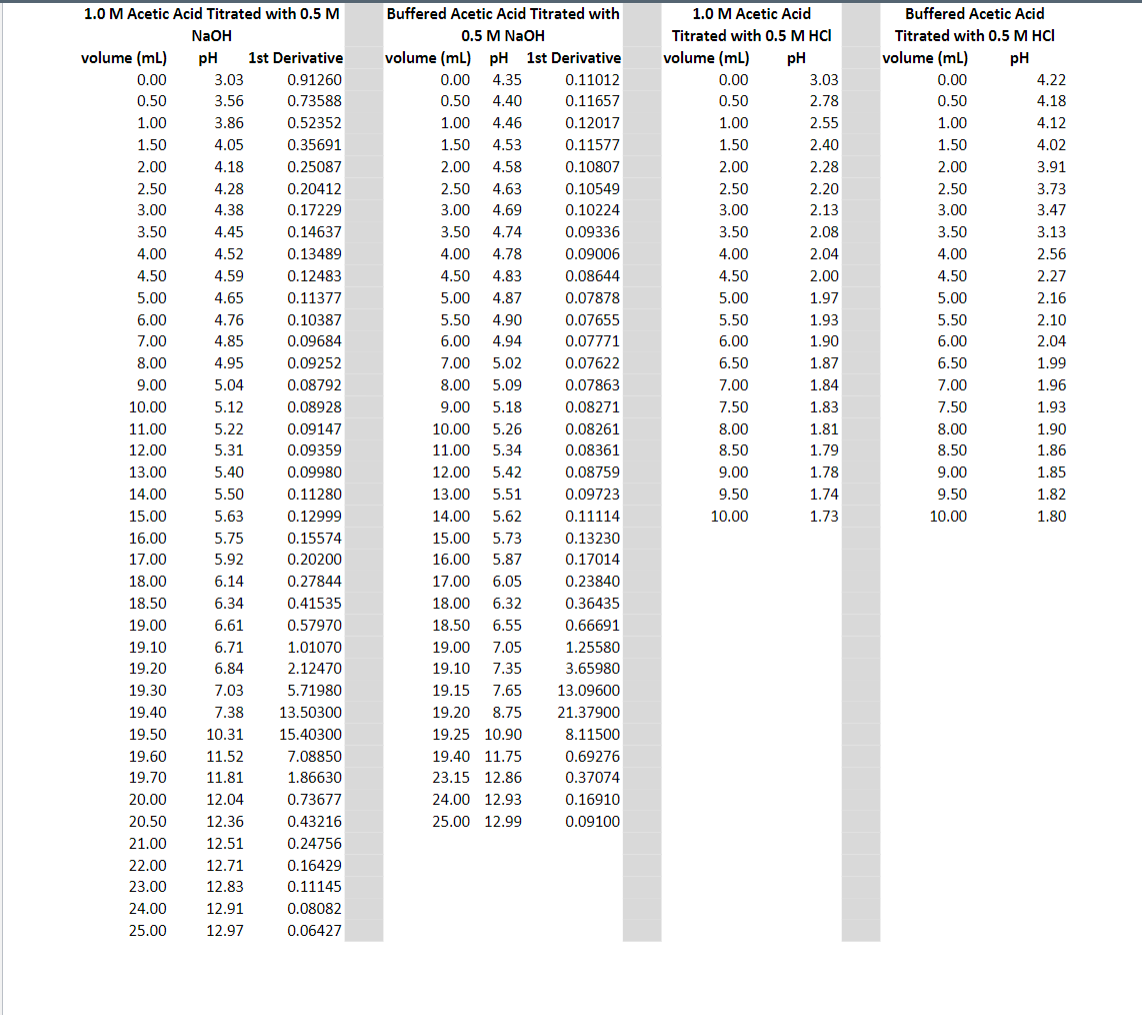
1) The beginning pH for the first plot is 3.03. Why is this so? Where did that number come from? 2) What is the initial pH for titration 2? (shown from data to be 4.35 pH) Where did that number come from? What species are present at the start of this titration? 3) For the third titration, you are in effect titrating sodium acetate (a weak base) with HCL, a strong acid. By recording how much HCl was needed, you can calculate the moles of sodium acetate used to prepare the buffered solution (1:1 ratio).
1.50 1.0 M Acetic Acid Titrated with 0.5 M NaOH volume (ml) pH 1st Derivative 0.00 3.03 0.91260 0.50 3.56 0.73588 1.00 3.86 0.52352 1.50 4.05 0.35691 2.00 4.18 0.25087 2.50 4.28 0.20412 3.00 4.38 0.17229 3.50 4.45 0.14637 4.00 4.52 0.13489 4.50 4.59 0.12483 5.00 4.65 0.11377 6.00 4.76 0.10387 7.00 4.85 0.09684 8.00 4.95 0.09252 9.00 5.04 0.08792 10.00 5.12 0.08928 11.00 5.22 0.09147 12.00 5.31 0.09359 13.00 5.40 0.09980 14.00 5.50 0.11280 15.00 5.63 0.12999 16.00 5.75 0.15574 17.00 5.92 0.20200 18.00 6.14 0.27844 18.50 6.34 0.41535 19.00 6.61 0.57970 19.10 6.71 1.01070 19.20 6.84 2.12470 19.30 7.03 5.71980 19.40 7.38 13.50300 19.50 10.31 15.40300 19.60 11.52 7.08850 19.70 11.81 1.86630 20.00 12.04 0.73677 20.50 12.36 0.43216 21.00 12.51 0.24756 22.00 12.71 0.16429 23.00 12.83 0.11145 24.00 12.91 0.08082 25.00 12.97 0.06427 Buffered Acetic Acid Titrated with 0.5 M NaOH volume (ml) pH ist Derivative 0.00 4.35 0.11012 0.50 4.40 0.11657 1.00 4.46 0.12017 1.50 4.53 0.11577 2.00 4.58 0.10807 2.50 4.63 0.10549 3.00 4.69 0.10224 3.50 4.74 0.09336 4.00 4.78 0.09006 4.50 4.83 0.08644 5.00 4.87 0.07878 5.50 4.90 0.07655 6.00 4.94 0.07771 7.00 5.02 0.07622 8.00 5.09 0.07863 9.00 5.18 0.08271 10.00 5.26 0.08261 11.00 5.34 0.08361 12.00 5.42 0.08759 13.00 5.51 0.09723 14.00 5.62 0.11114 15.00 5.73 0.13230 16.00 5.87 0.17014 17.00 6.05 0.23840 18.00 6.32 0.36435 18.50 6.55 0.66691 19.00 7.05 1.25580 19.10 7.35 3.65980 19.15 7.65 13.09600 19.20 8.75 21.37900 19.25 10.90 8.11500 19.40 11.75 0.69276 23.15 12.86 0.37074 24.00 12.93 0.16910 25.00 12.99 0.09100 1.0 M Acetic Acid Titrated with 0.5 M HCI volume (mL) pH 0.00 3.03 0.50 2.78 1.00 2.55 2.40 2.00 2.28 2.50 2.20 3.00 2.13 3.50 2.08 4.00 2.04 4.50 2.00 5.00 1.97 5.50 1.93 6.00 1.90 6.50 1.87 7.00 1.84 7.50 1.83 8.00 1.81 8.50 1.79 9.00 1.78 9.50 1.74 10.00 1.73 Buffered Acetic Acid Titrated with 0.5 M HCI volume (mL) pH 0.00 4.22 0.50 4.18 1.00 4.12 1.50 4.02 2.00 3.91 2.50 3.73 3.00 3.47 3.50 3.13 4.00 2.56 4.50 2.27 5.00 2.16 5.50 2.10 6.00 2.04 6.50 1.99 7.00 1.96 7.50 1.93 8.00 1.90 8.50 1.86 9.00 1.85 9.50 1.82 10.00 1.80
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


