Question: This will be the order in which the groups deprotonate during a titration, The most acidic group will also determine the initial pH of a
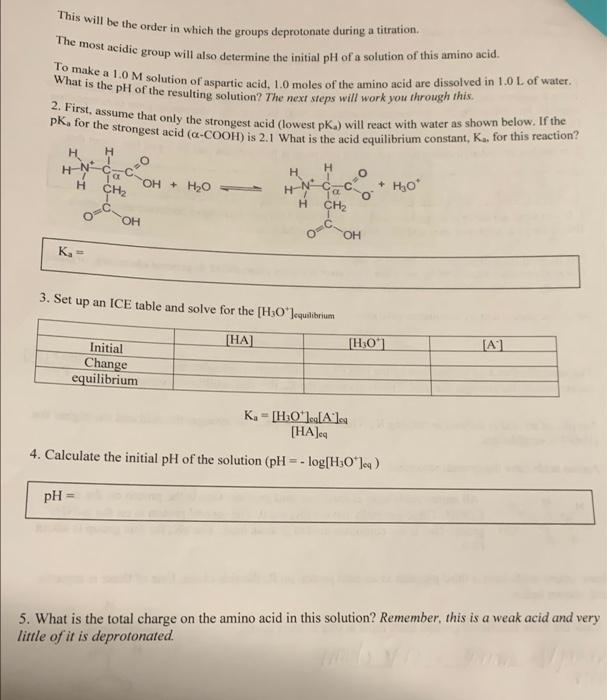
This will be the order in which the groups deprotonate during a titration, The most acidic group will also determine the initial pH of a solution of this amino acid. Wohnmoke M solution of aspartic acid , 1.0 moles of the amino acid are dissolved in 1.0 L of water. Bli fort has ume that only the strongest acid (lowest pk. ) will react with water as shown below. If the What is the pH of the resulting solution? The next steps will work you through this , for the strongest acid (-COOH) is 2.1 What is the acid equilibrium constant, K., for this reaction? H H H HN-d H H OH + H2O - + Hot H CH2 OH qoc O CH2 00 K- 3. Set up an ICE table and solve for the [H3O'lcquilibrium [HA] [H:01 [A Initial Change equilibrium Ka [HO']es[A]sa [HA] 4. Calculate the initial pH of the solution (pH = -log[H:0*) pH 5. What is the total charge on the amino acid in this solution? Remember, this is a weak acid and very little of it is deprotonated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


