Question: Tho reactant concentration in a zero-order reaction was 0.100M afler 175 s and 2.50x102M after 330 s . What is the rate constant for this

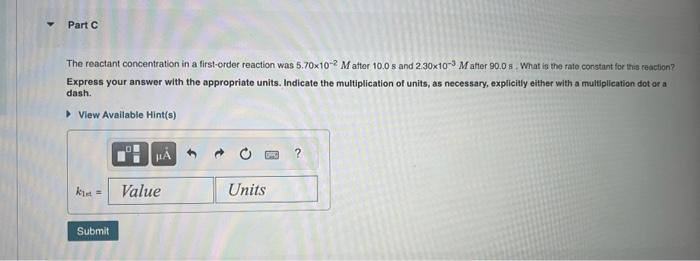
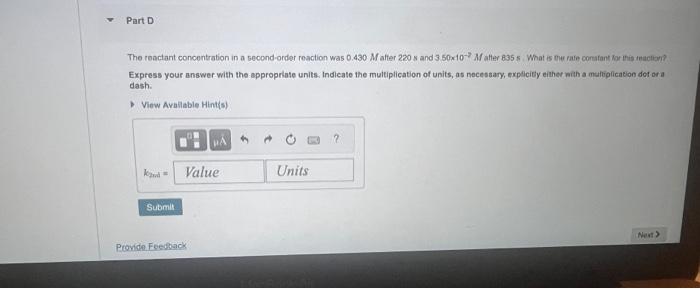
Tho reactant concentration in a zero-order reaction was 0.100M afler 175 s and 2.50x102M after 330 s . What is the rate constant for this reaction? Express your answer with the appropriate units. Indieate the multiplication of units, as necessary, explicity oither with a muttiplication dot or a dash. View Available Hint(s) The reactant concentration in a first-order reaction was 5.70102M after 10.05 and 2.30103M atter 90.08. What is the fato corstant for inis reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. The reactant concentration in a second-order reaction was 0.430M after 220s and 3.50102M after 835s. What is tee rate conatant for his teaction? Express your answer with the appropriate units. Indleate the multiplication of units, as necessary, explicity either with a multipfication der or a dash
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


