Question: Thorough steps please! And please explain how you convert the freezing point to Kf and how to get its cm to do so in order
 Thorough steps please! And please explain how you convert the freezing point to Kf and how to get its cm to do so in order to get to the answer?
Thorough steps please! And please explain how you convert the freezing point to Kf and how to get its cm to do so in order to get to the answer?
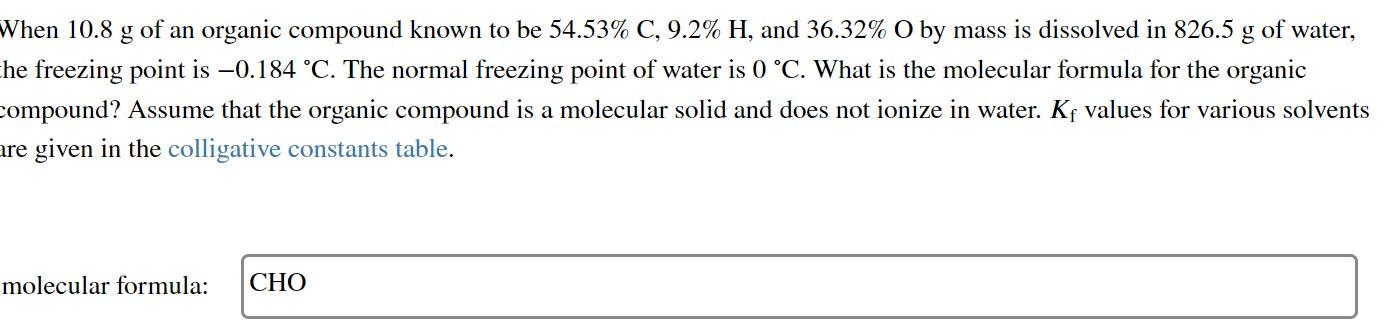
When 10.8g of an organic compound known to be 54.53%C,9.2%H, and 36.32%O by mass is dissolved in 826.5g of water, he freezing point is 0.184C. The normal freezing point of water is 0C. What is the molecular formula for the organic ompound? Assume that the organic compound is a molecular solid and does not ionize in water. Kf values for various solvents given in the colligative constants table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


