Question: Three proteins (A, B and C) are separated in a chromatography column using a buffer solution. The isotherms between the proteins in solution and the
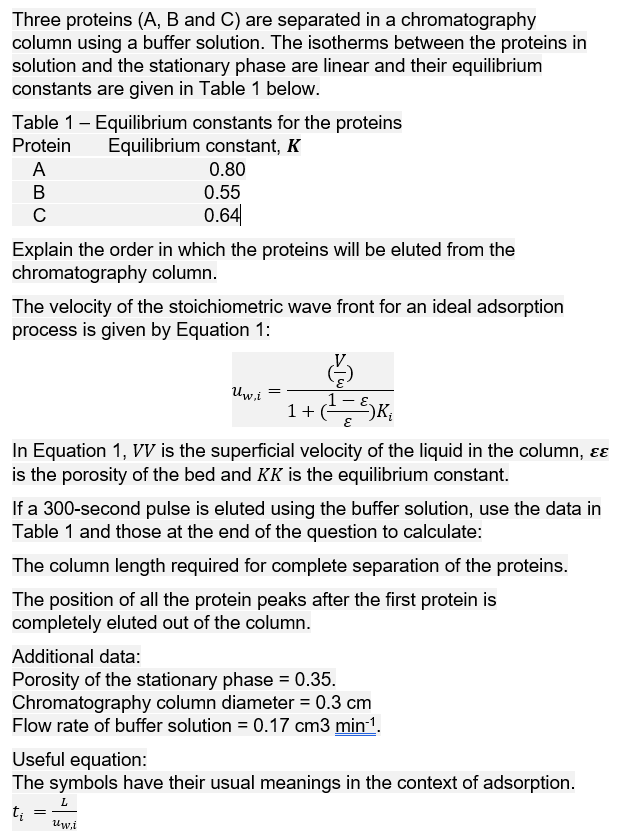
Three proteins (A, B and C) are separated in a chromatography column using a buffer solution. The isotherms between the proteins in solution and the stationary phase are linear and their equilibrium constants are given in Table 1 below. Table 1 - Equilibrium constants for the proteins Equilibrium constant, K Protein A 0.80 B 0.55 0.64 Explain the order in which the proteins will be eluted from the chromatography column. The velocity of the stoichiometric wave front for an ideal adsorption process is given by Equation 1: = Uw.i 1 + ( = ) K & Ki E In Equation 1, VV is the superficial velocity of the liquid in the column, is the porosity of the bed and KK is the equilibrium constant. If a 300-second pulse is eluted using the buffer solution, use the data in Table 1 and those at the end of the question to calculate: The column length required for complete separation of the proteins. The position of all the protein peaks after the first protein is completely eluted out of the column. Additional data: Porosity of the stationary phase = 0.35. Chromatography column diameter = 0.3 cm Flow rate of buffer solution = 0.17 cm3 min-. Useful equation: The symbols have their usual meanings in the context of adsorption. ti = uw.i
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


