Question: to access important values if needed for this question. ces The behaviors of four different solutes when dissolved in water are indicated by the four
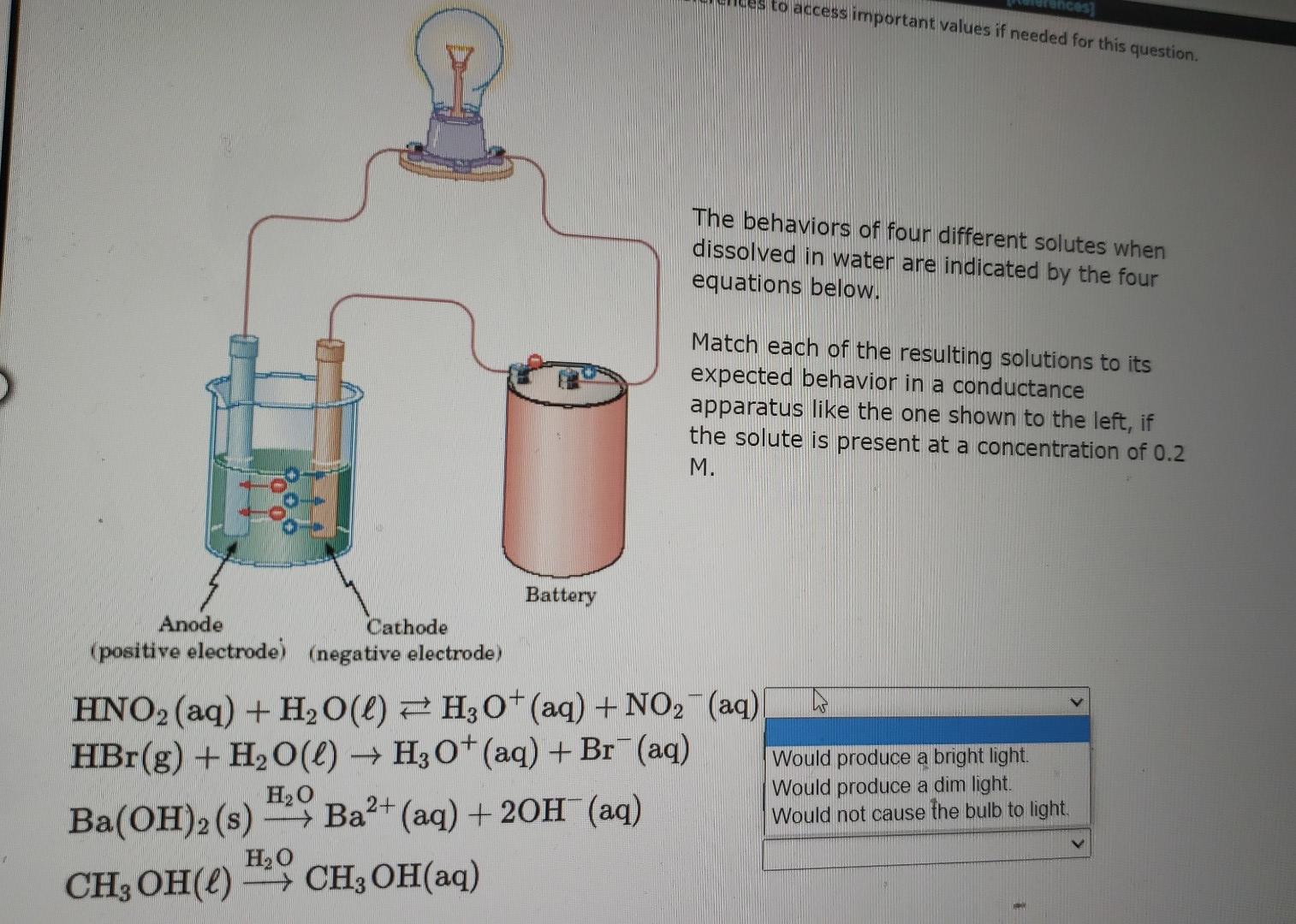
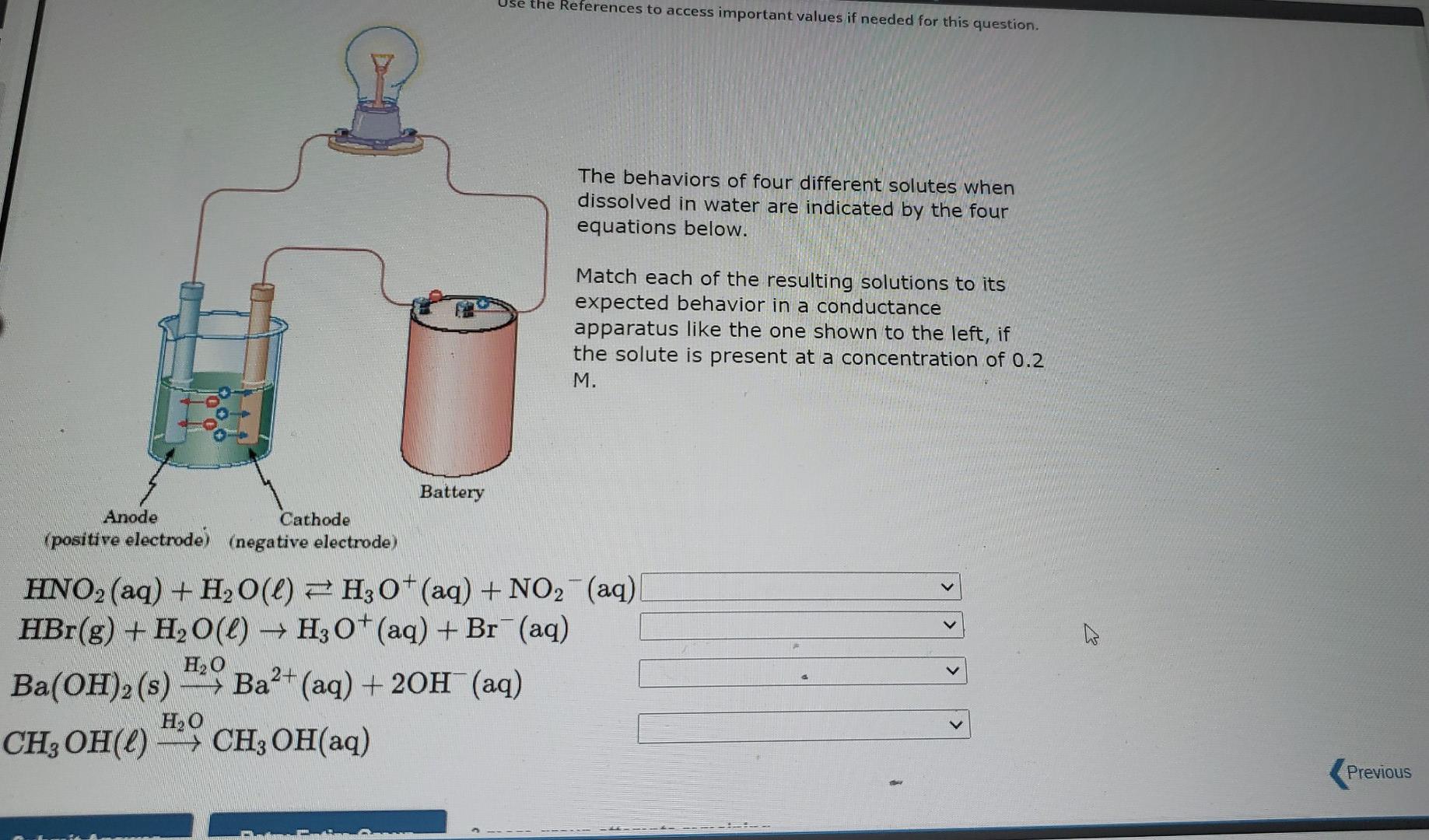
to access important values if needed for this question. ces The behaviors of four different solutes when dissolved in water are indicated by the four equations below. Match each of the resulting solutions to its expected behavior in a conductance apparatus like the one shown to the left, if the solute is present at a concentration of 0.2 M. Battery Anode Cathode (positive electrode) (negative electrode) -> HNO2 (aq) + H2O(l) 7 H3O+ (aq) + NO2 (aq) HBr(g) + H2O(l) + H30+ (aq) + Br+ (aq) Would produce a bright light. H20 Would produce a dim light. Ba(OH)2 (s) - Ba2+ (aq) + 2OH(aq) Would not cause the bulb to light H,0 CH, OH(L) - CH2OH(aq) Ba+ Use the References to access important values if needed for this question. The behaviors of four different solutes when dissolved in water are indicated by the four equations below. Match each of the resulting solutions to its expected behavior in a conductance apparatus like the one shown to the left, if the solute is present at a concentration of 0.2 M. Anode Battery Cathode (positive electrode) (negative electrode) HNO2 (aq) + H2O(l) FH, O+ (aq) + NO2 (aq) HBr(g) + H2O(l) Ho+(aq) + Br(aq) H2O Ba(OH)2(s) -- Ba2+ (aq) + 2OH(aq) ) > H20 CH3OH(L) > CH3 OH(aq) - Previous
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


