Question: To predict the change in urea concentration dur- ing the period between dialyses, the model describing the change in plasma levels of molecules removed
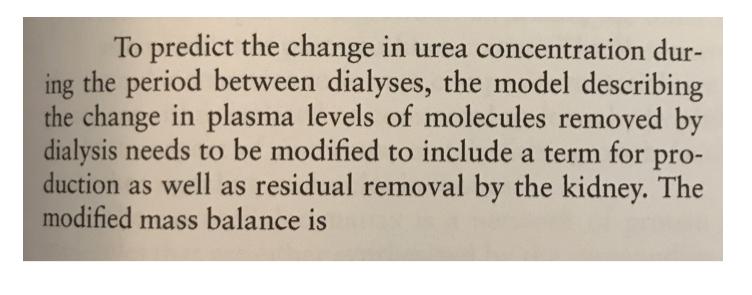

To predict the change in urea concentration dur- ing the period between dialyses, the model describing the change in plasma levels of molecules removed by dialysis needs to be modified to include a term for pro- duction as well as residual removal by the kidney. The modified mass balance is vdc, dt (7.11.5) where G is the constant production rate and KR is the residual clearance of the kidneys. = G (K + KR) Cis 1 (a) For G= 0.00022 mol min-, V = 40 L, KR = 6 mL min, K = 167 mL min-, and a initial urea con- centration of 37 mM, solve Equation (7.11.5) and determine the urea concentration after 4 hours of dialysis. Determine the effect of residual produc- tion and synthesis on the level of urea in the plasma during this 4-hour period of dialysis. (b) Now consider the rise in the urea concentration during the period between dialysis treatments. There is no clearance by dialysis (K = 0), but there is residual removal from the kidney and produc- tion. Modify the solution from part (a) to deter- mine the steady-state concentration and the time to reach steady state. (c) Calculate the dialysate concentration in blood at the beginning of the next dialysis, which is three days later. Determine the number of cycles for the concentrations until the concentration rise between cycles is the same. Give the final levels before and after dialysis.
Step by Step Solution
3.31 Rating (151 Votes )
There are 3 Steps involved in it
To solve this problem we will apply the principles of a mass balance on the system described in the question The equation given is V fracdCidt G K KR ... View full answer

Get step-by-step solutions from verified subject matter experts


