Question: transport phenomena2 3. Consider the biosensor device shown in the figure in the below. The biosensor is designed to measure the concentration of solute A
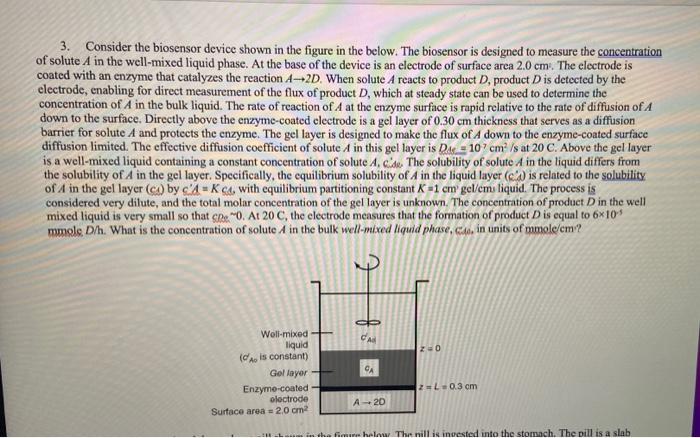
3. Consider the biosensor device shown in the figure in the below. The biosensor is designed to measure the concentration of solute A in the well-mixed liquid phase. At the base of the device is an electrode of surface area 2.0cm. The electrode is coated with an enzyme that catalyzes the reaction A2D. When solute A reacts to product D, product D is detected by the electrode, enabling for direct measurement of the flux of product D, which at steady state can be used to determine the concentration of A in the bulk liquid. The rate of reaction of A at the enzyme surface is rapid relative to the rate of diffusion of A down to the surface. Directly above the enzyme-coated electrode is a gel layer of 0.30cm thickness that serves as a diffusion barrier for solute A and protects the enzyme. The gel layer is designed to make the flux of A down to the enzyme-coated surface diffusion limited. The effective diffusion coefficient of solute A in this gel layer is Der=107cm2/s at 20C. Above the gel layer is a well-mixed liquid containing a constant concentration of solute A, cide. The solubility of solute A in the liquid differs from the solubility of A in the gel layer. Specifically, the equilibrium solubility of A in the liquid layer ( A ) is related to the solubility of A in the gel layer (c) by cA=KCA, with equilibrium partitioning constant K=1cm ' gel/cmu liquid. The process is considered very dilute, and the total molar concentration of the gel layer is unknown. The concentration of product D in the well mixed liquid is very small so that cDe2. At 20C, the electrode measures that the formation of product D is equal to 6105 mmole D/h. What is the concentration of solute A in the bulk well-mixed liquid phase, Csd, in units of mmole/cm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


