Question: Two aqueous sulfuric acid solutions containing 20.0wt%H_(2)SO_(4)(SG ) = ( 1.139) and 60.0wt%H_(2)SO_(4)(SG ) = ( 1.498) are mixed to form a 4.00 molar solution
Two aqueous sulfuric acid solutions containing
20.0wt%H_(2)SO_(4)(SG)
=(
1.139)and
60.0wt%H_(2)SO_(4)(SG)
=(
1.498)are mixed to form a 4.00 molar solution )
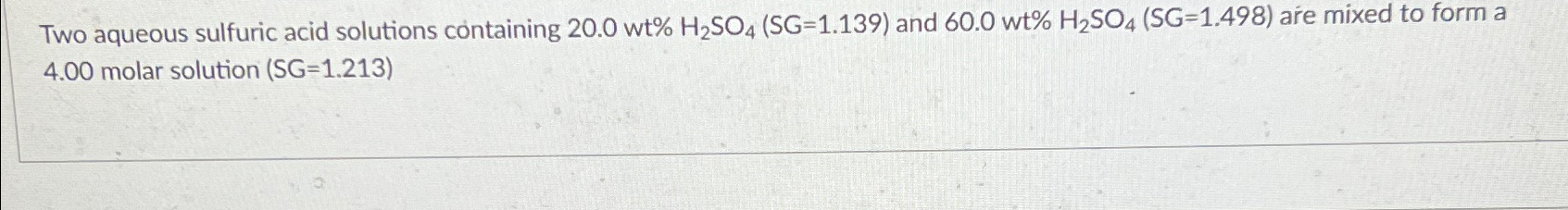
Two aqueous sulfuric acid solutions containing 20.0wt%H2SO4(SG=1.139) and 60.0wt%H2SO4(SG=1.498) are mixed to form a 4.00 molar solution (SG=1.213 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


