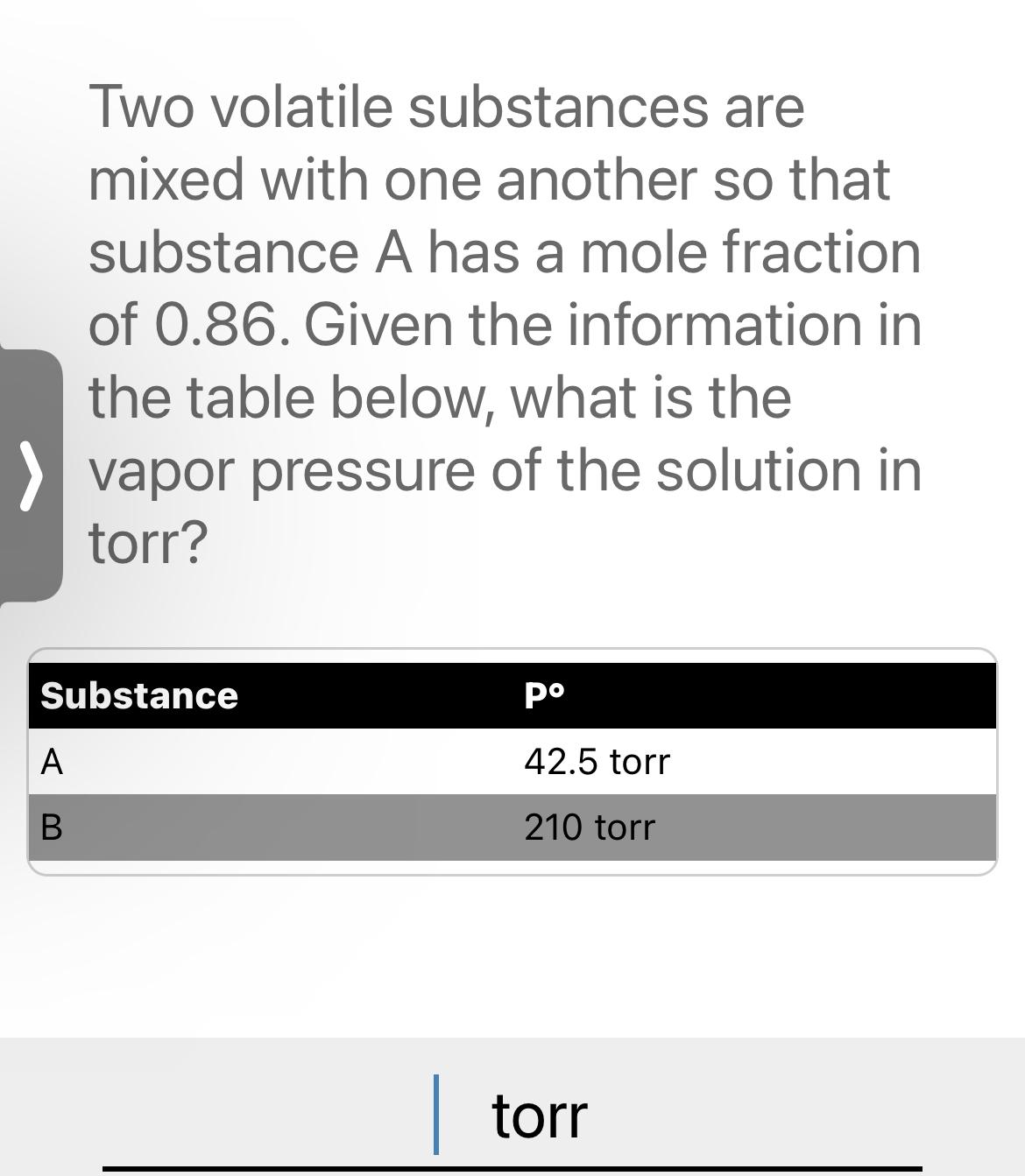
Question: Two volatile substances are mixed with one another so that substance A has a mole fraction of 0 . 8 6 . Given the information
Two volatile substances are mixed with one another so that substance A has a mole fraction of Given the information in the table below, what is the vapor pressure of the solution in torr?
tableSubstancepoA torrB torr
torr
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


