Question: Two volatile substances are mixed with one another so that the solution has a vapor pressure of 125 torr. Given the information in the table
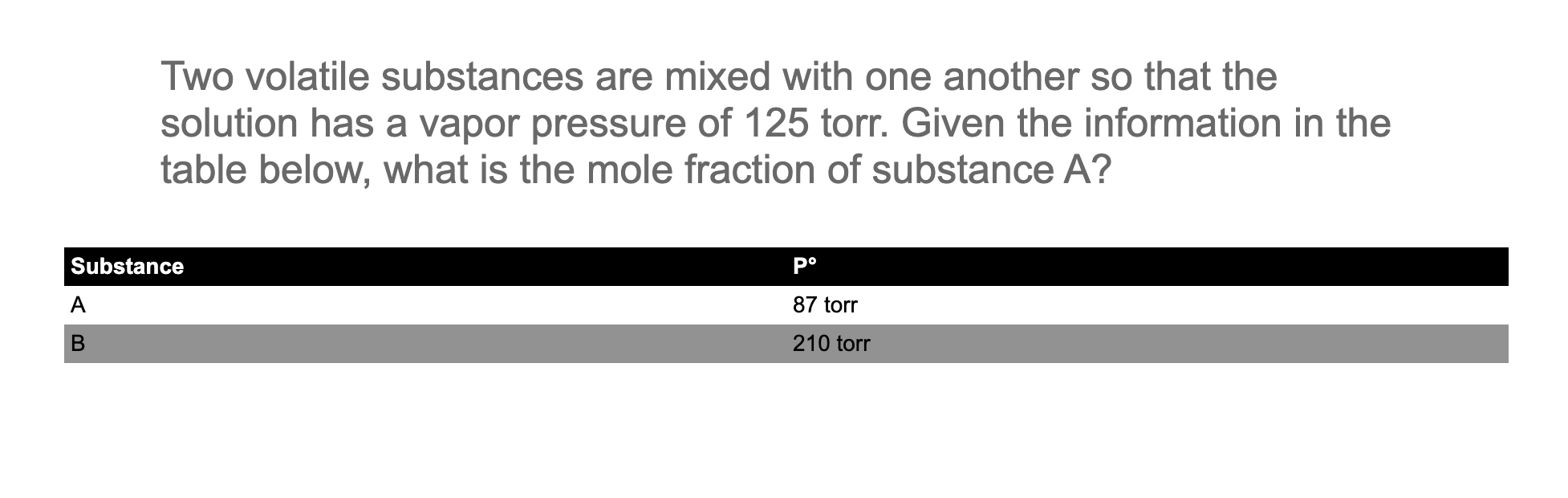
Two volatile substances are mixed with one another so that the solution has a vapor pressure of 125 torr. Given the information in the table below, what is the mole fraction of substance A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


