Question: ume air is a 20/80 split between oxygen and nitrogen. Answer the following questions: a. Write a balanced stoichiometric equation for this reaction. b. Estimate
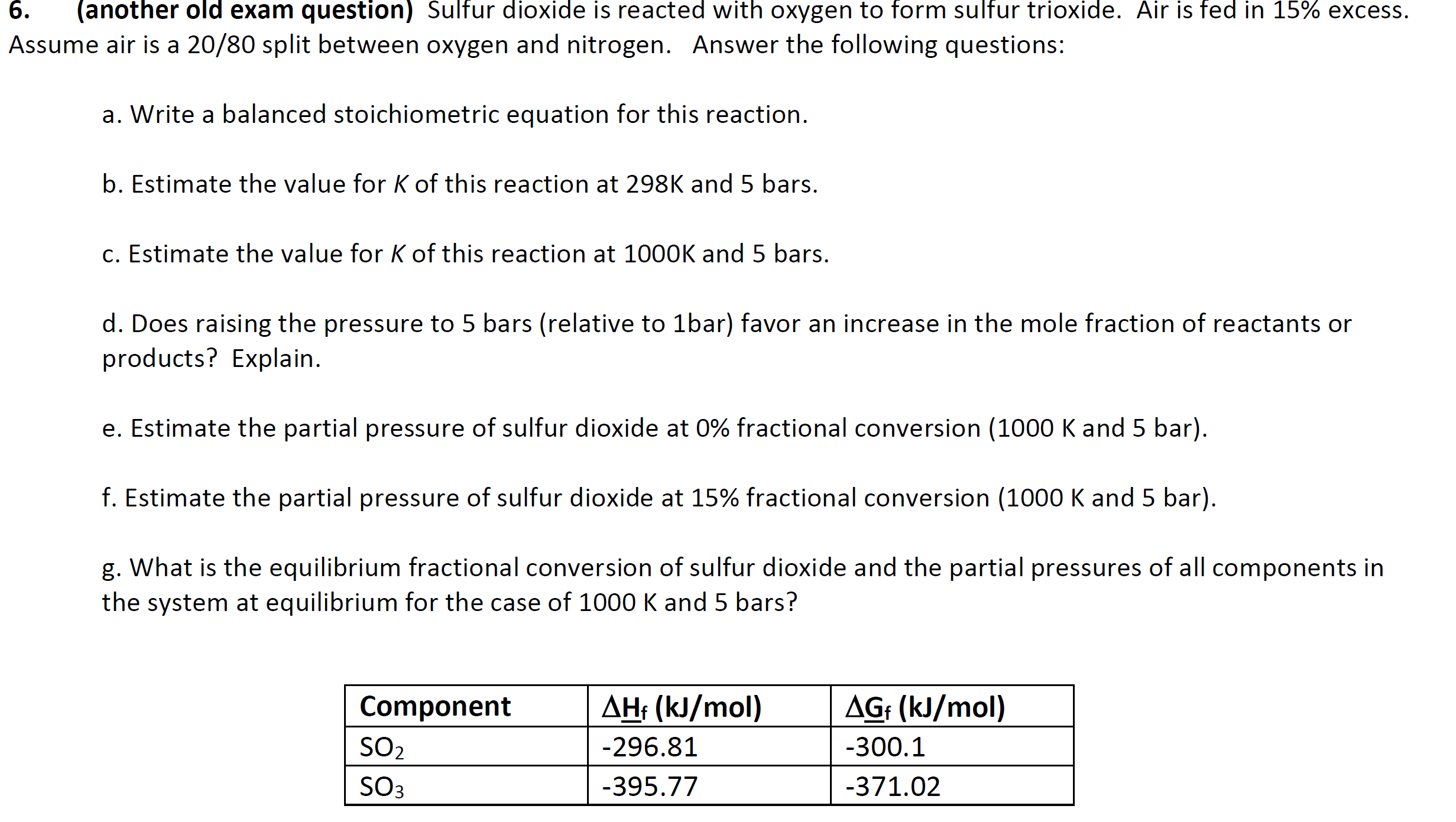
ume air is a 20/80 split between oxygen and nitrogen. Answer the following questions: a. Write a balanced stoichiometric equation for this reaction. b. Estimate the value for K of this reaction at 298K and 5 bars. c. Estimate the value for K of this reaction at 1000K and 5 bars. d. Does raising the pressure to 5 bars (relative to 1 bar) favor an increase in the mole fraction of reactants or products? Explain. e. Estimate the partial pressure of sulfur dioxide at 0% fractional conversion (1000 K and 5 bar). f. Estimate the partial pressure of sulfur dioxide at 15% fractional conversion (1000 K and 5 bar). g. What is the equilibrium fractional conversion of sulfur dioxide and the partial pressures of all components in the system at equilibrium for the case of 1000K and 5 bars
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


