Question: Use an Arrhenius plot to determine the frequency factor for the reaction to two sig figs. (please answer part B, 3.5 is incorrect) The following
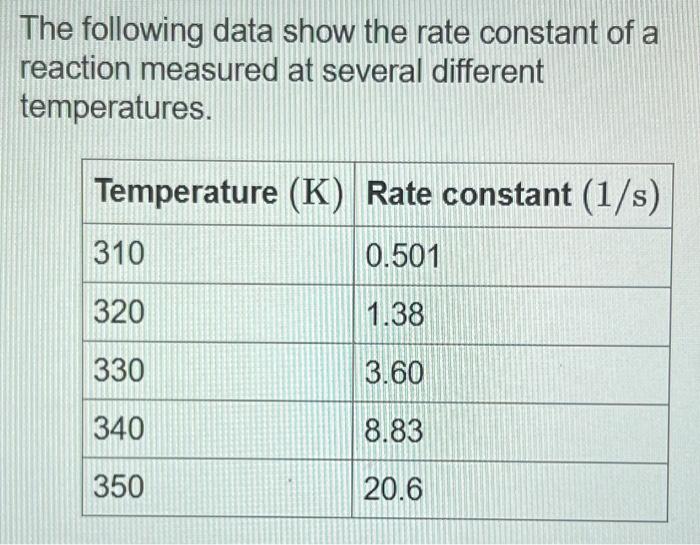
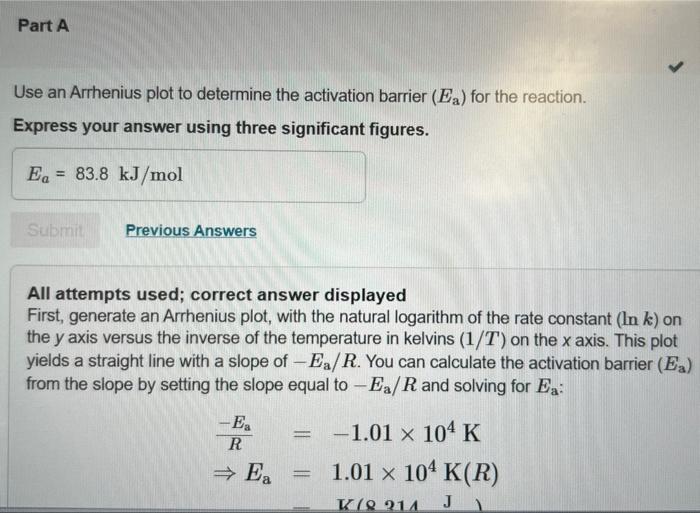
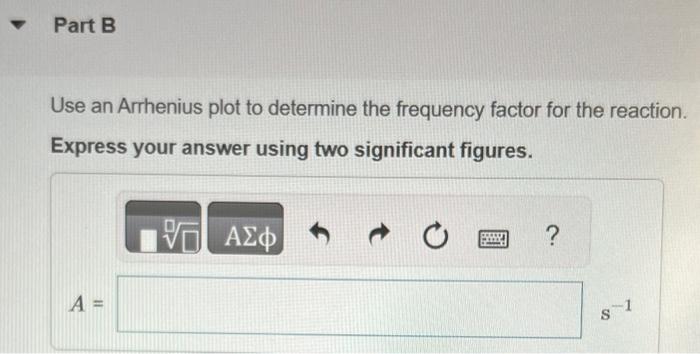
The following data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier (Ea) for the reaction. Express your answer using three significant figures. All attempts used; correct answer displayed First, generate an Arrhenius plot, with the natural logarithm of the rate constant (lnk) on the y axis versus the inverse of the temperature in kelvins (1/T) on the x axis. This plot yields a straight line with a slope of Ea/R. You can calculate the activation barrier (Ea) from the slope by setting the slope equal to Ea/R and solving for Ea : REa=1.01104KEa=1.01104K(R) Use an Arrhenius plot to determine the frequency factor for the reaction. Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


