Question: Use Given data. Table 2 Given, will thumbs up if correct Use the Clausius-Clapeyron equation to determine the normal boiling point of water. Refer to

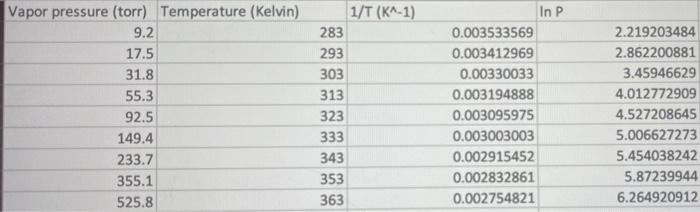
Use the Clausius-Clapeyron equation to determine the normal boiling point of water. Refer to Table 2 on your data sheet and pick any data point to use for P1 and T1. Use the known heat of vaporization of 44.01 kJ/mole. Show your work below. An unknown liquid has a heat of vaporization of 8.17 kJ/mole. If the vapor pressure of this liquid at -181.4 C is 100. torr, what is the normal boiling point of this liquid? Vapor pressure (torr) Temperature (Kelvin) 9.2 17.5 31.8 55.3 92.5 149.4 233.7 355.1 525.8 1/T (KA-1) 283 293 303 313 323 333 343 353 In P 0.003533569 0.003412969 0.00330033 0.003194888 0.003095975 0.003003003 0.002915452 0.002832861 0.002754821 2.219203484 2.862200881 3.45946629 4.012772909 4.527208645 5.006627273 5.454038242 5.87239944 6.264920912 363
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


