Question: use Matlab and write out the code. answer all part A to C or I will give you a thumbs down and report you Problem
use Matlab and write out the code. answer all part A to C or I will give you a thumbs down and report you
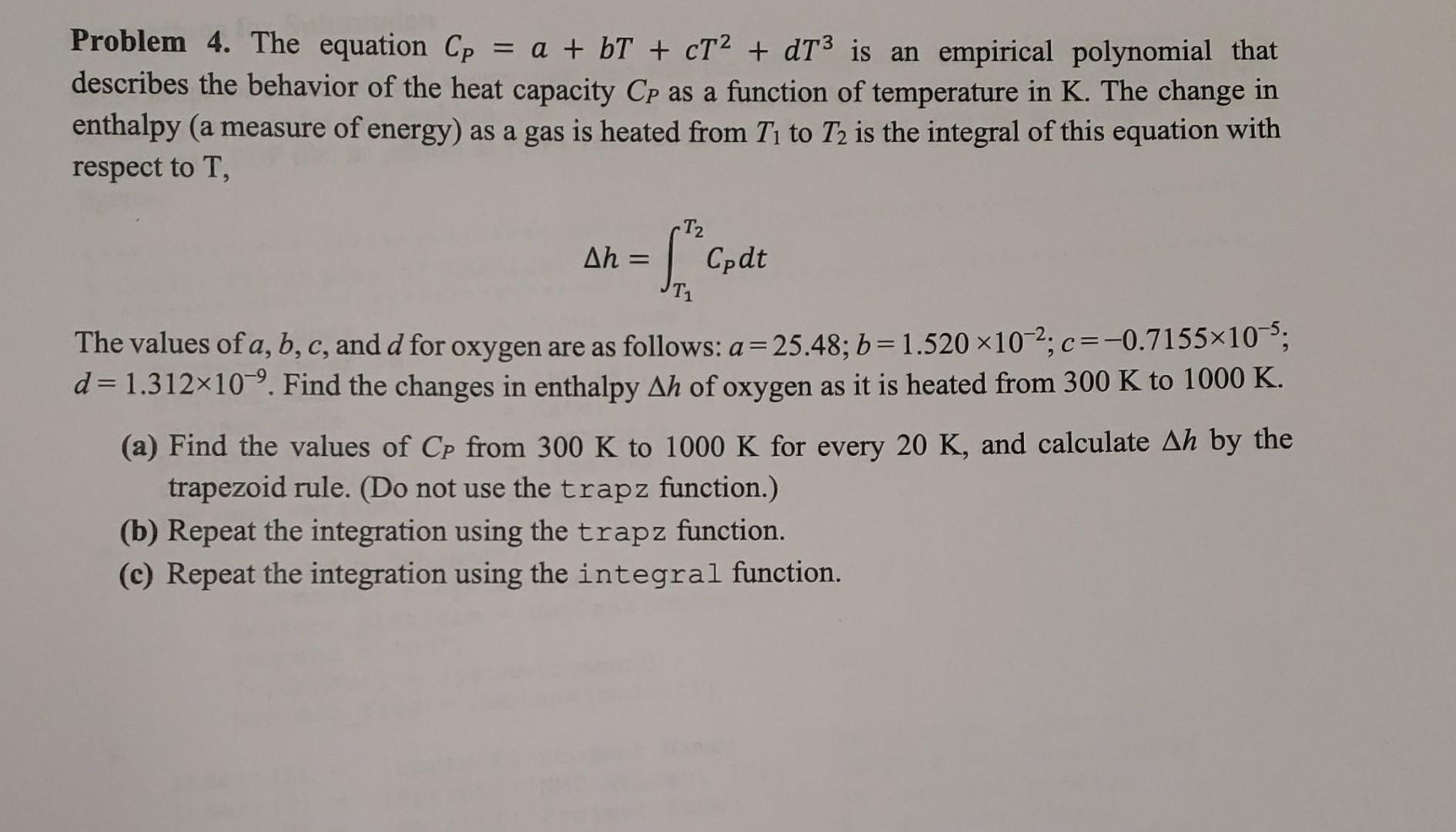
Problem 4. The equation CP=a+bT+cT2+dT3 is an empirical polynomial that describes the behavior of the heat capacity CP as a function of temperature in K. The change in enthalpy (a measure of energy) as a gas is heated from T1 to T2 is the integral of this equation with respect to T, h=T1T2CPdt The values of a,b,c, and d for oxygen are as follows: a=25.48;b=1.520102;c=0.7155105; d=1.312109. Find the changes in enthalpy h of oxygen as it is heated from 300K to 1000K. (a) Find the values of CP from 300K to 1000K for every 20K, and calculate h by the trapezoid rule. (Do not use the trapz function.) (b) Repeat the integration using the trapz function. (c) Repeat the integration using the integral function
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


