Question: use Matlab and write out the code. answer all part A to C or I will give you a thumbs down and report you that
use Matlab and write out the code. answer all part A to C or I will give you a thumbs down and report you
that is all the information given please answer all parts A to C
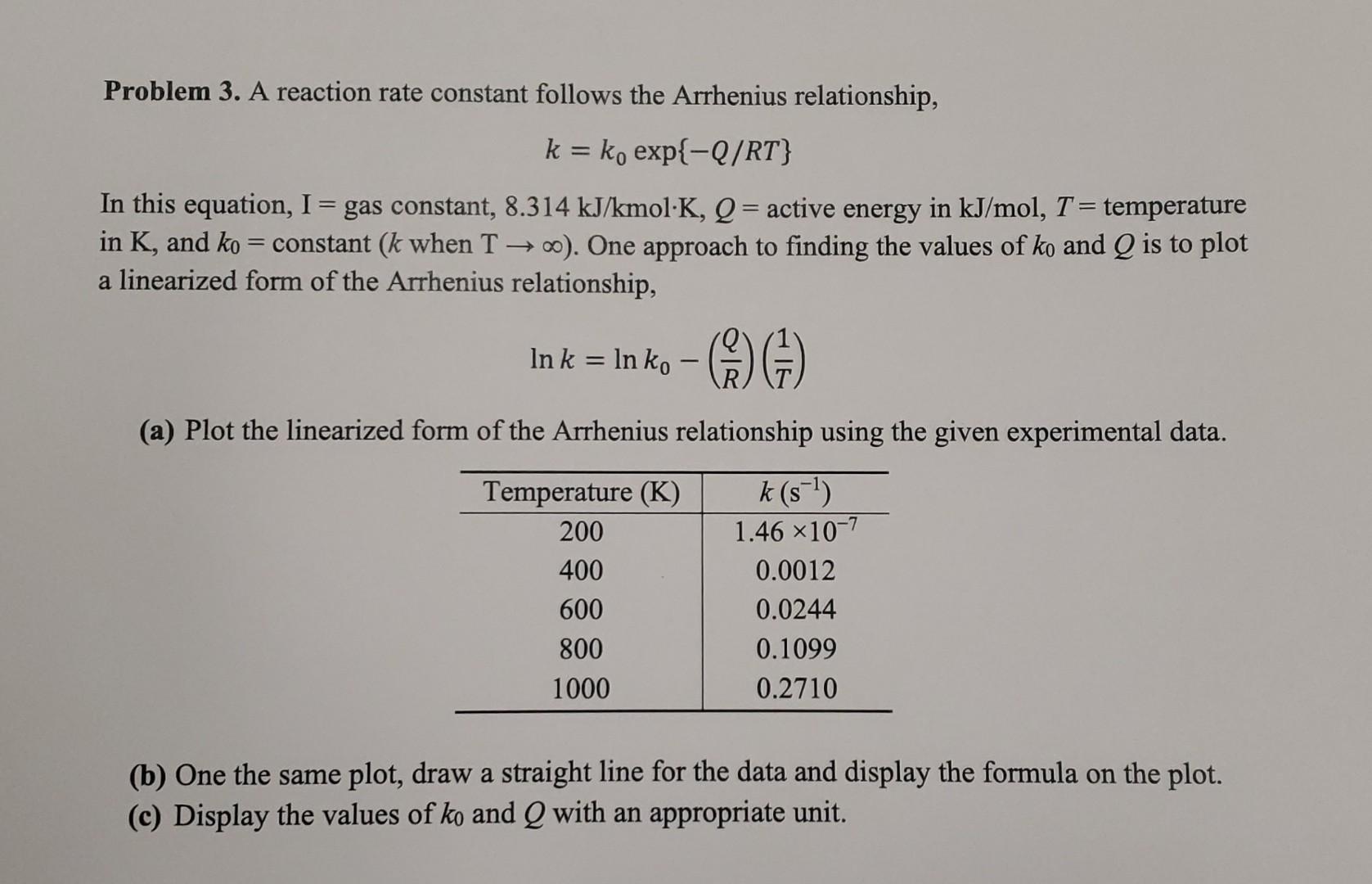
Problem 3. A reaction rate constant follows the Arrhenius relationship, k=k0exp{Q/RT} In this equation, I= gas constant, 8.314kJ/kmolK,Q= active energy in kJ/mol,T= temperature in K, and k0= constant (k when T ). One approach to finding the values of k0 and Q is to plot a linearized form of the Arrhenius relationship, lnk=lnk0(RQ)(T1) (a) Plot the linearized form of the Arrhenius relationship using the given experimental data. (b) One the same plot, draw a straight line for the data and display the formula on the plot. (c) Display the values of k0 and Q with an appropriate unit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


