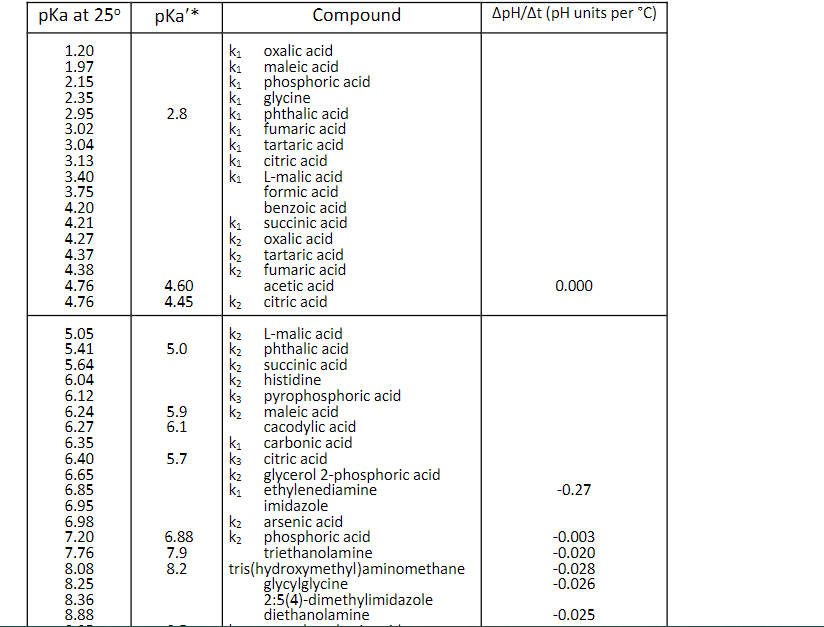
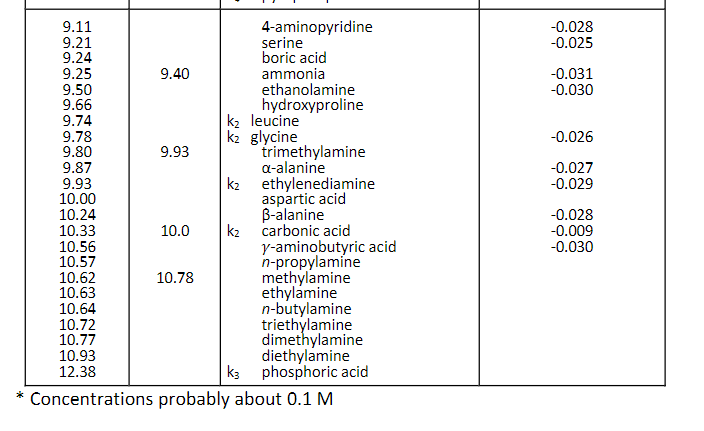
Question: Use pka to solve the problem, not pka'*. Use pKa (not pKa') from Table 2-1 in the coursepack. Calculate your answers to three (3) significant
Use pka to solve the problem, not pka'*.
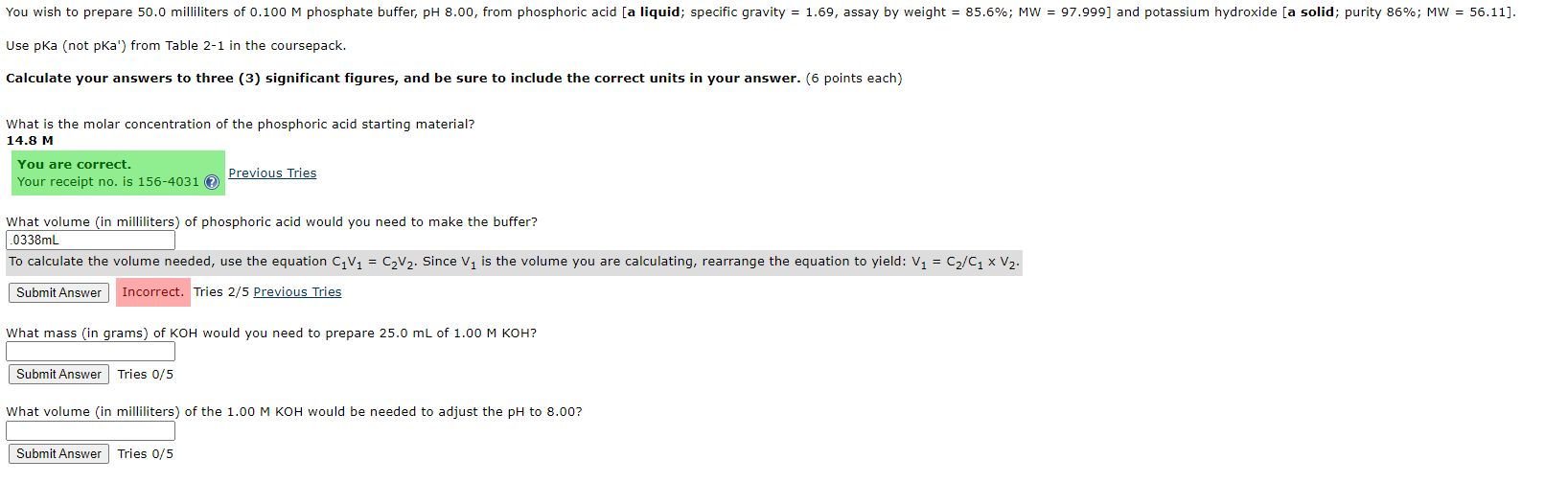
Use pKa (not pKa') from Table 2-1 in the coursepack. Calculate your answers to three (3) significant figures, and be sure to include the correct units in your answer. (6 points each) What is the molar concentration of the phosphoric acid starting material? 14.8 M Previous Tries What volme (in milliliters) of phosphoric acid would you need to make the buffer? .0338mL To calculate the volume needed, use the equation C1V1=C2V2. Since v1 is the volume you are calculating, rearrange the equation to yield: V1=C2/C1V2. Tries 2/5 Previous Tries What mass (in qrams) of KOH would you need to prepare 25.0mL of 1.00MKOH ? Tries 0/5 What volume (in milliliters) of the 1.00MKOH would be needed to adjust the pH to 8.00 ? Tries 0/5 * Concentrations probably about 0.1M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


