Question: Use the data in the table below collected from squid to answer the following discussion questions. Extracellular Concentration Ion Relative Permeability Na CI K+
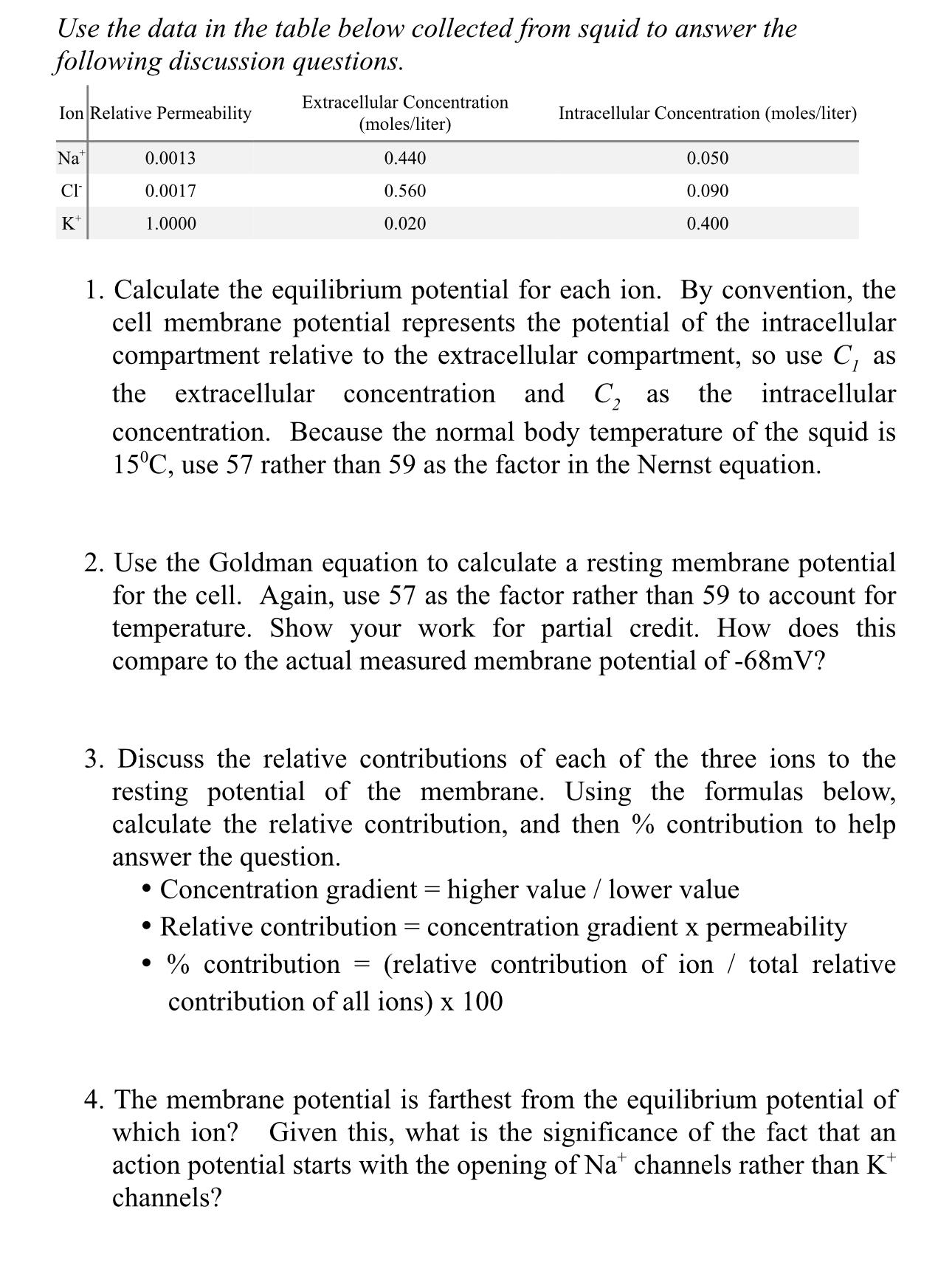
Use the data in the table below collected from squid to answer the following discussion questions. Extracellular Concentration Ion Relative Permeability Na CI K+ 0.0013 0.0017 1.0000 (moles/liter) 0.440 0.560 0.020 Intracellular Concentration (moles/liter) 1. Calculate the equilibrium potential for each ion. By convention, the cell membrane potential represents the potential of the intracellular compartment relative to the extracellular compartment, so use C, as the extracellular concentration and C as the intracellular concentration. Because the normal body temperature of the squid is 15C, use 57 rather than 59 as the factor in the Nernst equation. 0.050 0.090 0.400 2. Use the Goldman equation to calculate a resting membrane potential for the cell. Again, use 57 as the factor rather than 59 to account for temperature. Show your work for partial credit. How does this compare to the actual measured membrane potential of -68mV? 3. Discuss the relative contributions of each of the three ions to the resting potential of the membrane. Using the formulas below, calculate the relative contribution, and then % contribution to help answer the question. = Concentration gradient = higher value / lower value Relative contribution concentration gradient x permeability % contribution (relative contribution of ion / total relative contribution of all ions) x 100 = 4. The membrane potential is farthest from the equilibrium potential of which ion? Given this, what is the significance of the fact that an action potential starts with the opening of Nat channels rather than K* channels?
Step by Step Solution
There are 3 Steps involved in it
Lets go through each question step by step 1 To calculate the equilibrium potential for each ion we can use the Nernst equation Since the temperature ... View full answer

Get step-by-step solutions from verified subject matter experts


